 |
SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS |
| << MICROEMULSION TECHNIQUES:Significance of Packing Parameter |
| NEWER SYNTHETIC STRATERGIES FOR NANOMATERIALS:Nanostructured Materials >> |
Chapter
- 8
SYNTHESIS
BY SOLID STATE
DECOMPOSITION
S.
Navaladian
INTRODUCTION
Synthesis
of materials is an important part in
materials science and chemistry
because the
properties
of materials vary drastically
based on their synthetic
method. Even the
stability
of
the material varies based on
method by which it is synthesized. By
varying synthetic
methods,
surface area, pore size,
crysatallite size, allotropes,
morphology, presence of
impurities,
defects and other oxidation state of
the metal can also be varied.
Hence, their
potential
to certain applications vary
drastically. For example, in
catalysis, catalyst
with
high
surface area is preferred to
achieve high conversion. So,
synthetic method that
yield
high
surface of the catalyst is adopted. A
lot of methods are known in
the synthesis of
materials
of various applications in different
fields, still efficient and
cost-effective
methods
are being developed.
DECOMPOSITION
METHODS
Decomposition
is one of the methods known in
the synthesis of various
materials.
In
this
method,
a solid or liquid is heated to
its decomposition temperatures to obtain
the solid of
interest.
For example, sugar can be decomposed in
an inert atmosphere to get the
carbon
material
(solid-to-solid). Decomposition of
titanium isopropoxide (a liquid) in
air
atmosphere
is used to get the titanium
dioxide (TiO2)
(liquid-to-solid). Ni(CO)4, a
volatile
complex
can be decomposed to get Ni metal
(vapour-to-solid). In this particular
chapter,
solid
state decomposition is considered.
Solid-State
Decomposition
Decomposition
of any material occurs due to
its instability when the
particular conditions
are
applied. Also, it depends
upon the activating source. These
activating sources can be
thermal,
photochemical, microwave and γ-radiation.
For example chemical
compound
which
need high temperatures to decompose can
be photochemically decomposed
with
ease
at room temperature. Particularly,
the decomposition of AgBr to Ag can be
achieved
by
thermal modes only at 1330�C
but it can be easily decomposed at
room temperature
under
visible light. As we know,
this principle is used in
photography. Thus, based on
the
activating
sources, solid-state decomposition can be
classified into the
following
8.2
Synthesis
by Solid State
Decomposition
decomposition
methods (1) thermal
decomposition, (2) Microwave-assisted and
(3)
Photochemical
decomposition.
Thermal
Decomposition Methods
In
general, thermal decomposition
methods are chosen for
synthesis of metal
oxides.
Decomposable
precursors which posses low
decomposition temperature are
preferred for
the
synthesis because at high
temperatures, sintering is also high. So
materials formed
will
have poor surface area and
large crystallite size.
Decomposition temperature of
any
compounds
is mainly decided by the
redox properties of the
compound. In general, salts
of
the silver with easily
utilizable organic moiety can be
decomposed at lower
temperatures.
Various materials such as
metal, metal oxides, mixed
metal oxides and
metals
chalogenides have been synthesized
using decomposition
method.
Synthesis
of Metals
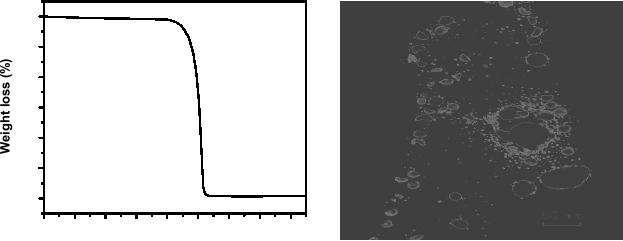
100
a
b
95
90
85
80
75
70
40
60
80
100 120 140 160
180 200
ο
Temperature
( C )
Fig.
8.1. TGA profile of silver
oxalate and (b) silver
nanoparticles synthesized by
decomposition
of silver oxalate
[1].
Synthesis
of metallic silver can be achieved
using silver formate, silver
oxalate, oleate,
maleate
and fumarate and their decomposition
temperature is 93, 140, 287,
170 and 280
�C
respectively (1 and 2). Recently
silver dodecanate, myristate and
palmitate also have
been
utilized for the synthesis
of silver nanopartices. Decomposition of
these silver
compounds
yield various gaseous
products like CO, CO2 and
organic residues. In the
case
of silver oxalate decomposition to Ag
metal and CO2 are
produced. Hence, this
is
one
of the methods known for
the production pure CO2. In
the case of silver
oxalate
Synthetic
Strategies in Chemistry
8.3
decomposition,
reaction was carried out in a
water medium under refluxing
conditions at
under
N2 atmosphere
with a capping agent (poly
(vinyl alcohol)) (Fig. 8.1).
TGA of the
silver
oxalate, given in Fig. 8.1
(a) shows the sharp
decomposition at around 140 �C.
The
corresponding
weight loss is due to the loss of two
moles of CO2.
TEM image of Ag
nanoparticles
synthesized by decomposition of silver
oxalate is given in Fig.
8.1(b). The
average
particle is around 7
nm.
Metal
Oxides
Metal
oxides can also be synthesized by
decomposition of certain precursors
like
hydroxides,
oxalates, hydroxyl carbonates, hydroxyl
sulphates and carbonates. In
general,
the
direct calcination of metal
salt such as nitrates and
sulphates is not carried out
to
synthesis
the metal oxides in order to
avoid the impurities and
heterogeity of the
materials.
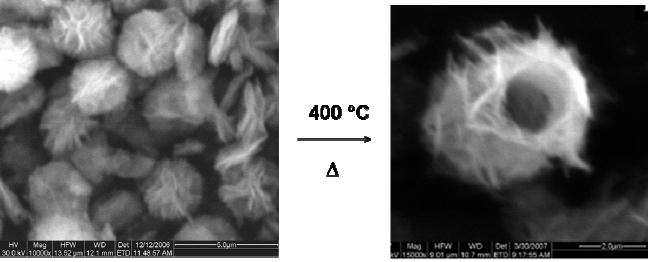
Synthesis
of Magnesia
The
decomposition of magnesium
hydroxycarbonate (Mg5(CO3)4(OH)2)
yields
magnesium
oxide (MgO). In this
particular case, the
morphology of the precursor
is
flower
like particle of 3 �m. After
calcinations at 400 �C, MgO
with tube-like
morphology
is obtained as shown in Fig.
8.2.
ο
Mg5 (CO3 )4 (OH)2 400→
5
MgO +
4CO2 + H2O
C
(a)
(b)
Fig.
8.2 SEM image of (a)
magnesium hydroxycarbonate and (a)
MgO [3].

8.4
Synthesis
by Solid State
Decomposition
Similarly
MgSO4.5Mg(OH)2.2H2O
also yields the MgO by
decomposition, but
the
complete
decomposition needs temperature above 800
�C.
In this case, morphology
of
the
particles is strip-like. In general,
MgO is synthesized decomposing the
Mg(OH)2 in
the
air. But MgO synthesized in
the presence of air shows
less surface area than
that of
MgO
synthesized in nitrogen atmosphere. This
is due to the high amount of
sintering in
presence
of oxygen.
Synthesis
of Matel Oxides from Oxalate
Precursors
Due
to the low temperature
decomposition of metal oxalates,
oxalate precursors are
most
widely
used for syntheses of metal
oxides. Nickel,
copper, iron and zinc oxides
can be
synthesized
directly decomposing their
oxalate precursor. Nickel
oxalate precursor has
yielded
nickel oxide with sizes
around 9 nm at 450 �C. Fe2O3 has
been synthesized by
thermal
decomposition of ferrous oxalate at 415
�C.
When copper oxalate has
been
decomposed
in air at 300 �C for 1 h
mesoporous CuO microspheres have been
obtained
(Fig.
8.3). Zinc oxalate has
yielded ZnO particles of size
around 55 nm. In all these
cases,
the
formation of CO and CO2 are
the by products. Zinc
acetate also decomposes and
yields
the zinc oxide at 600 �C.
Stoichiometric barium titanate
(BaTiO3)
is synthesized
by
thermal decomposition of bariumtitanyl
oxalate at 600 �C.
Fig.
8.3. Copper oxide micropshere
obtained by the decomposition of copper
oxalate [4].
Synthetic
Strategies in Chemistry
8.5
Metal
Chalcogenides
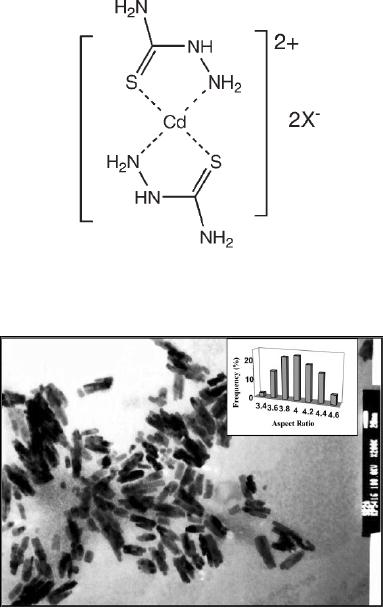
Cd(II)complex
of thiosemicarbazide and selenosemicarbazide are
thermally decomposed
to
obtain CdS and CdSe. In
typical procedure 500 mg of cadmium
tetramethylthiourea
complex
was mixed with 182 mg of
thiosemicarbazide ligand, dispersed
well in 3 ml tri-
n-octylphosphine
oxide (TOPO). This precursor
mixture was injected into
TOPO (5 g) at
300
�C.
The resulting orange yellow
solution was maintained at 300 �C for
about 1 h and
then
cooled to 70 �C. TEM image of
CdS nanorods formed are
shown in Fig. 8.4. In
this
case
the thicknesses of the rods formed
are 15 nm and aspect ratio is
3-4.
Cadmium
thiosemicarbazide
Fig.
8.4. CdS nanorods
synthesized by decomposition of cadmium
thiosemicarbazide [5]
8.6
Synthesis
by Solid State
Decomposition
Microwave-assisted
Decomposition
In
general, microwave is used to
quicken the reactions. The
main advantages of
microwave
assisted processes are: (i)
the rate of the reaction is
increased by orders of
magnitude,
(ii) the initial heating
process is rapid, and (iii)
microwaves induce the
generation
of localized high temperatures at
reaction sites, which enhances
the reaction
rates.
Moreover, microwave-based syntheses are
energy efficient. Hence,
microwave is
used
in the synthesis of materials as
well as organic
compounds.
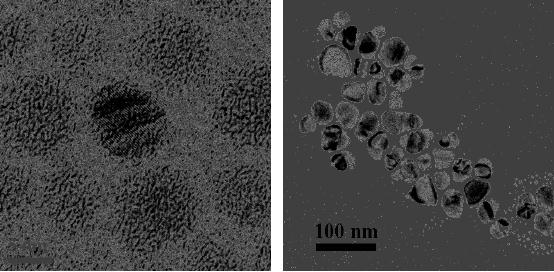
Synthesis
of Metals
Decomposition
of silver oxalate has been
carried out by using
microwave radiation in
ethylene
glycol and diethylene glycol.
Formation of silver nanoparticles
has been
observed
in 60 s in the case of ethylene
glycol. In the case of
diethyelene glycol,
only
after
75 s of irradiation, the formation of
silver nanoparticles has
been observed. Ag
nanoparticle
formed in ethylene glycol
medium is given in Fig. 8.5
(a). Formation of
anisotropic
nanoparticle is observed in the case of
75 s of microwave irradiation (see
for
example
Fig. 8.5 (a)).
a
b
Fig.
8.5. TEM images of Ag nanoparticles
formed in (a) 60 s and (b) 75 s of
microwave
irradiation
[6].
Synthesis
of Metal Oxides
CaMoO4 has been synthesized at
low temperatures by a modified
citrate complex
method
using
microwave irradiation. Synthesizing
mixed metal oxides at low
temperatures is a
Synthetic
Strategies in Chemistry
8.7
tedious
process an it needs high
temperatures. As a result, the
resulting material has
poor
surface
area and larger crystallite
size. For this kind of
purpose, microwave can be used
to
effectively bring down the
temperature required for the
formation of mixed
metal
oxides.
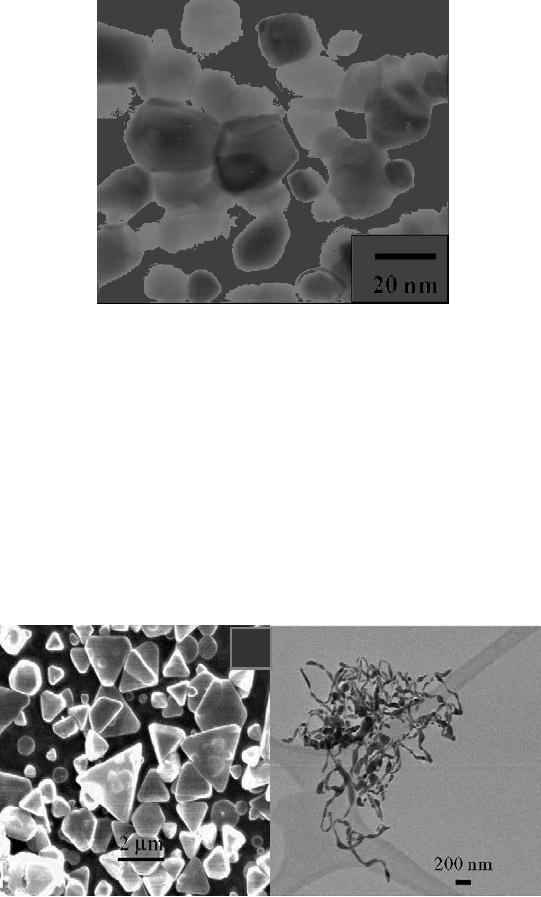
A flow chart for the
synthesis of CaMoO4 is
given Scheme 8.1. In this
case, citric
acid
makes a complex with the
metal ions and due to the
presence of the citrate,
the
formation
of the nanoparticles is observed. This
method is also known as citric
acid
combustion
method. The corresponding
nanoparticles of CaMoO4 formed
are shown in
Fig.
8.6.
Scheme
8.1. Flow chart of the
synthesis of CaMoO4 by
microwave assisted route
[9]
8.8
Synthesis
by Solid State
Decomposition
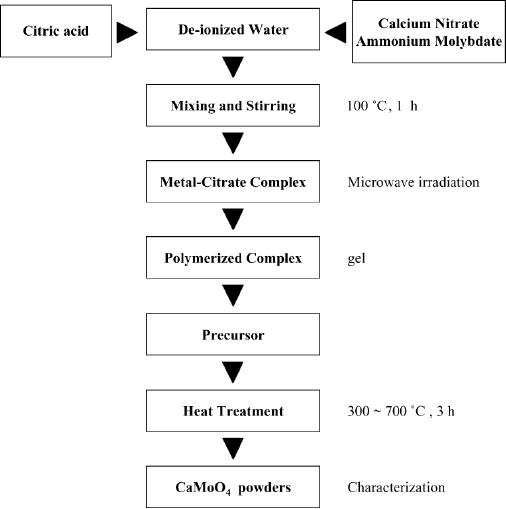
Fig.
8.6. TEM image of CaMoO4 nanoparticle
synthesized at 600 �C for 3 h
[9]
Photochemical
Decomposition Method
Synthesis
of Metals
Silver
nanowires have been
synthesized using photographic
principles. In this case,
AgBr
is
reduced by in presence of a fluorescent
light and followed by the
development of the
film.
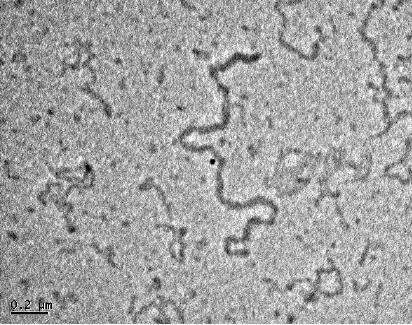
TEM images AgBr and Ag nanowires
formed from this process
are shown in Fig
8.7.
a
b
Fig.
8.7. TEM images of (a) AgBr
and (b) Ag nanowire
[10]
Synthetic
Strategies in Chemistry
8.9
Synthesis
of Metal Oxides
Iron
oxide Nanoparticles
Nanoparticles
of iron oxide have been
synthesized by decomposing
Fe(CO)5 under
ultraviolet
light. During the
photolysis, decomposition of
Fe(CO)5 takes
place to form
nanoparticles
of iron metal and further it
turns into iron oxide due to
the instability of
iron
nanoparticles.
Synthesis
of EuO nanocrystals
In
a quartz vessel, Eu(NO3)3 (37.5
mM) and urea (112.5 mM) were dissolved in
methanol
(400
ml) under an N2 atmosphere,
and the solution was irradiated
with a 500-W high-
pressure
mercury arc lamp at 25�C. A
yellowish powder precipitated
after 30 min. After
24
h of irradiation, the powder was
separated by centrifugation and washed
with
methanol
several times.
Synthesis
of CdS nanorod
Fig.
8.8. TEM image of CdS nanowires
synthesized by photochemical method
[11].
CdS
nanorods have been
synthesized using cadmium
salt, DNA base pairs and
thioacetamide
(TAA). Photo-irradiation was performed by
directly placing the
cuvette
containing
the mixture of DNA, Cd salt,
and TAA solutions under 260 nm UV
light
8.10
Synthesis
by Solid State
Decomposition
irradiation
for six hours. The
initially occurring
DNA-Cd2+ complex formation
was
confirmed
by a shift in the UV-vis
spectrum of the mixed
solution compared to the
pure
DNA.
After photo-irradiation the
UV-vis spectrum was again recorded
showing the
formation
of CdS nanoparticles. Thioacetamide
acts as sulphur source in this
reaction.
The
corresponding CdS nanowires
formed are shown in TEM image in
Fig. 8.8.
SUMMARY
Solid
state decomposition methods
are important in the
synthesis of the material
both in
nano
size ranges as well as in bulk. In
the case of thermal
decomposition route,
the
compound
having lower decomposition
temperature is preferred. The
decomposition
temperature
of the compound is mainly
based on the redox potential
of cation and anion
of
the compound. Microwave-assisted
syntheses are more advantageous
than
conventional
heating due to the increased rate of
the reaction. Photochemical
synthetic
methods
are also advantageous due to fact that
the material is formed at
ambient
conditions
so that the sintering of the
materials is reduced.
REFERENCES
1.
S.
Navaladian, B. Viswanathan, R. P.
Viswanath and T. K. Varadarajan,
Nanoscale
Research Letter,
2
(2007) 44.
I.
K. Shim, Y. L. Lee, K. J. Lee, J. Joung, Materials
Chemistry and
Physics,
2.
(2008)
(in press).
3.
C.
M. Janet, B. Viswanathan, R. P. Viswanath and T. K.
Varadarajan, Journal
of
Physical
Chemistry C,
111 (2007) 10267.
4.
R.
N. Nickolov,
B.
V. Donkova, K. I. Milenova1 and D. R.
Mehandjiev,
Adsorption
Science & Technology,
24 (2006) 497.
5.
P.
S. Nair, T. Radhakrishnan, N. Revaprasadu, G. A.
Kolawole and P.
O'Brien,Chemiacl
Communication (2002)
564.
6.
S.
Navaladian, C. M. Janet, B. Viswanathan, R. P.
Viswanath and T. K.
Varadarajan,
Nanotechnology, 19 (2008) 045603.
7.
X.
Wang, J. Song, L. Gao, J. Jin, H.
Zheng and Z. Zhang, Nanotechnology
16
(2005)
37.
8.
Z.
Zhou, Q. Sun, Z. Hu, and Y.
Deng, Journal
of Physical Chemistry C,
110
(2006)
13387.
Synthetic
Strategies in Chemistry
8.11
9.
J.
H. Ryu, J.-W. Yoon, C. S.
Lim, W.-C. Oh and K. B. Shim,
Journal
of Alloys
and
Compounds, 390 (2005)
245.
10.
C.
Liewhiran, S. Seraphin, S. Phanichphant,
Current Applied Physics, 6
(2006)
499.
11.
S.
Liu, R. J. Wehmschulte, G. Lian and C. M.
Burba, Journal
of Solid State
Chemistry, 179
(2006) 696.
Table of Contents:
- INTRODUCTION TO SYNTHETIC STRATEGIES IN CHEMISTRY:POROUS MATERIALS
- SYNTHETIC METHODS BASED ON ACTIVATING THE REACTANT:HALOGENATION OF BENZENE
- METHODS BASED ON ACTIVATING THE REACTING SUBSTANCE:Experimental method
- SYNTHESIS OF MATERIALS BASED ON SOLUBILITY PRINCIPLE
- SOL-GEL TECHNIQUES:DEFINITIONS, GENERAL MECHANISM, INORGANIC ROUTE
- TEMPLATE BASED SYNTHESISSynthesis, Mechanism and Pathway
- MICROEMULSION TECHNIQUES:Significance of Packing Parameter
- SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS
- NEWER SYNTHETIC STRATERGIES FOR NANOMATERIALS:Nanostructured Materials
- THE ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY:The Holy Bible
- ELECTROCHEMICAL SYNTHESIS:FEATURES OF ELECTROCHEMICAL SYNTHESIS
- NEWER REACTIONS AND PROCEDURES: CATALYTIC AND NONCATALYTIC
- SYNTHETIC STRATEGIES - FROM LABORATORY TO INDUSTRY
- SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice
- CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES
- SOME CONCEPTUAL DEVELOPMENTS IN SYNTHESIS IN CHEMISTRY
- COMPUTATIONAL BASICS UNDERLYING SYNTHETIC STRATEGIES