 |
SOME CONCEPTUAL DEVELOPMENTS IN SYNTHESIS IN CHEMISTRY |
| << CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES |
| COMPUTATIONAL BASICS UNDERLYING SYNTHETIC STRATEGIES >> |
Chapter
- 16
SOME
CONCEPTUAL DEVELOPMENTS IN SYNTHESIS
IN
CHEMISTRY
Joyce
DSouza
INTRODUCTION
Synthesis
of materials play a crucial
role in designing and discovering
new materials and
also
in providing better and less cumbersome
methods for preparing known
chemicals.
Most
of the ten million or so
chemical compounds that are
known today, can be
classified
into
a relatively small number of
subgroups or families.
More
than 90 percent of the
compounds are organic
compounds. In turn,
organic
compounds
can be further subdivided into a
few dozen major families
such as the
alkanes,
alkenes, alkynes, alcohols, aldehydes,
ketones, carboxylic acids, and amines.
An
important
subset of organic compounds
include biochemical compounds
with four major
families:
carbohydrates, proteins, nucleic acids,
and lipids.
Because
of their unique properties,
multi-carbon compounds exhibit
extremely large
variety
and the range of application of organic
compounds is enormous. They
form the
basis
of important constituents of many
products and apart from a very
few exceptions,
they
form the basis of all
earthly life processes. The
different shapes and
chemical
reactivities
of organic molecules provide an
astonishing variety of functions,
like those of
enzyme
catalysts in biochemical reactions of
live systems. The autopropagating
nature of
these
organic chemicals is what
life is all about. Products
such as plastics,
synthetic
fibres,food,
explosives, paints and pigments,
pharmaceuticals and pesticides and
many
others
have become readily
available through the
dynamic development of
organic
synthesis.
Inorganic
compounds are typically
classified into one of five
major groups: acids,
bases,
salts, oxides, and others. A
large class of compounds
discussed in inorganic
chemistry
textbooks are coordination
compounds. Examples range from
species that are
strictly
inorganic, such as
[Co(NH3)6]Cl3,
to organometallic compounds such
as
Fe(C5H5)2 (ferrocene)and
extending to bioinorganic compounds,
such as the hydrogenase
enzymes.
Major classes of inorganic
compounds that are studied
under materials
science
tend
to be polymeric (non-molecular) and
refractory, and often are of
commercial interest
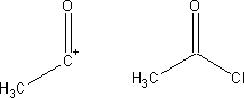
16.2
Some
Conceptual Developments in Synthesis in
Chemistry
such
as a)alloys: brass, bronze, stainless
steel; b)semiconductors : silicon ,
gallium
arsenide;
c)superconductors : yttrium barium copper
oxide .
SYNTHETIC
ORGANIC CHEMISTRY
Synthetic
organic chemistry is an applied
science as it borders engineering
involving
design,
analysis, and/or construction of
work for practical purposes.
Organic synthesis of
a
novel compound is a problem
solving task, where a
synthesis is designed for a
target
molecule
by selecting optimal reactions from
optimal starting materials.
Complex
compounds
can have several reaction
steps that sequentially
build the desired
molecule.
The
synthesis proceeds by utilizing
the reactivity of the
functional groups in
the
molecule.
For example, a carbonyl
compound can be used as a nucleophile by
converting
it
into an enolate, or as an electrophile,
and the combination of the
two is called the
aldol
reaction.
Designing practically useful syntheses
always requires conducting
the actual
synthesis
in the laboratory. The
scientific practice of creating
novel synthetic routes
for
complex
molecules is called total
synthesis.
There
are several strategies to design a
synthesis. The modern method
of
retrosynthesis,
developed by E.J. Corey, starts with
the target molecule and splices it
to
pieces
according to known reactions.
The pieces, or the proposed
precursors, are
further
spliced
until ideally inexpensive and
available starting materials
are reached. Then,
the
retrosynthesis
is written in the opposite direction to
give the synthesis. A
"synthetic tree"
can
be constructed, because each
compound and also each precursor
have multiple
syntheses.
Retrosynthetic
Analysis
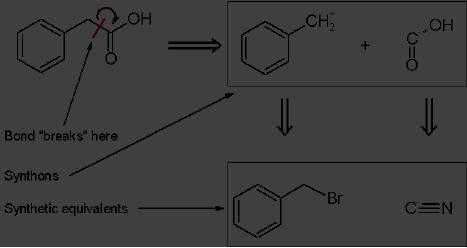
Disconnection:
A retrosynthetic step involving
the breaking of a bond to
form two (or
more)
synthons.
Synthon
Synthetic
equivalent
Synthetic
Strategies in Chemistry
16.3
Retron:
A minimal molecular substructure
that enables certain
transformations.
Retrosynthetic
tree: A directed acyclic
graph of several (or all)
possible retrosyntheses of
a
single target.
Synthon:
An idealized molecular fragment. A
synthon and the
corresponding
commercially
available synthetic equivalent
are shown below:
Target:
The desired final
compound.
Transform:
The exact reverse of a synthetic
reaction; the formation of
starting materials
from
a single product.
An
example will allow the
concept of retrosynthetic analysis to be
easily understood.
In
planning the synthesis of
phenylacetic acid, two
synthons are identified.
A
nucleophilic
"-COOH" group, and an electrophilic
"PhCH2+"
group. Of course, both
synthons
do not exist per se;
synthetic equivalents corresponding to
the synthons are
reacted
to produce the desired
product. In this case, the
cyanide anion is the
synthetic
equivalent
for the -COOH
synthon, while benzyl
bromide is the synthetic
equivalent for
the
benzyl synthon.
The
synthesis of phenylacetylene determined
by retrosynthetic analysis is
thus:
1.
PhCH2Br
+ NaCN → PhCH2CN +
NaBr
2.
PhCH2CN
+ 2 H2O → PhCH2COOH + NH3

16.4
Some
Conceptual Developments in Synthesis in
Chemistry
STRATEGIES
Functional
Group Strategies:
Manipulation of functional groups can
lead to significant
reductions
in molecular complexity.
Stereochemical
Strategies:
Numerous chemical targets have
distinct stereochemical
demands.
Stereochemical transformations (such as
the Claisen rearrangement
and
Mitsunobu
reaction) can remove or transfer
the desired chirality thus
simplifying the
target.
Structure-Goal
Strategies: Directing
a synthesis towards a desirable
intermediate can
greatly
narrow the focus of an
analysis. This allows
bidirectional search
techniques.
Transform-based
Strategies:
The application of transformations to
retrosynthetic
analysis
can lead to powerful reductions in
molecular complexity.
Unfortunately,
powerful
transform-based retrons are
rarely present in complex molecules, and
additional
synthetic
steps are often needed to
establish their
presence.
Topological
Strategies:
The identification of one or more
key bond disconnections
may
lead
to the identification of key
substructures : Disconnections that
preserve ring
structures
are encouraged; Disconnections
that create rings larger
than 7 members are
discouraged.
SYNTHETIC
INORGANIC CHEMISTRY
Advances
in inorganic chemistry have
made significant contributions to
modern living.
For
instance, synthetic fertilizers
manufactured from inorganic
chemicals have
increased
worldwide
crop production. Inorganic
substances used to fabricate
silicon chips help
power
the global information age.
Metal alloys are used in
automobiles and aircraft to
make
them lighter and stronger. Companies
use inorganic compounds to
fabricate
concrete,
steel, and glass--materials used to
construct buildings, infrastructure, and
other
public
works around the world. In
the United States, 10 of the
11 most commonly
produced
chemicals are derived from
inorganic elements. These 10 inorganic
chemicals
(presented
below in descending order of production)
are used in a wide variety
of
applications.
Sulfuric acid is used to make
fertilizers, synthetic fibers, and
metals.
Synthetic
Strategies in Chemistry
16.5
Nitrogen
is used in recovering underground
petroleum deposits, in the production
of
ammonia,
and as a blanketing material for
shipping perishables such as fruits
and
vegetables.
Oxygen is used in the
production of steel and plastics, in
medical
applications,
and in rocketry. Lime is used in
the manufacture of steel and
cement.
Ammonia
is combined with sulfuric acid to make
ammonium sulfate the most
important
of
the synthetic fertilizers.
Sodium hydroxide is used in
the manufacture of paper,
soap,
detergents,
and synthetic fibers, and is also a
caustic material used as a
drain cleaner.
Chlorine
is used to manufacture vinyl
chloride plastic, to disinfect
drinking water, and to
bleach
paper during manufacturing.
Phosphoric acid is used to give
soft drinks a tart
flavor
and to make fertilizers. Sodium carbonate is
used in the production of
glass, paper,
and
textiles. Nitric acid is used to make
synthetic fibers, such as
nylon; explosives,
such
as
nitroglycerin and TNT (trinitrotoluene); and is also
combined with ammonia to
make
fertilizer.
Because
of its direct relevance to
products of commerce, solid
state inorganic
chemistry
has been strongly driven by
technology. Applications discovered in
the 20th
century
include zeolite and platinum-based
catalysts for petroleum processing in
the
1950's,
high-purity silicon as a core component
of microelectronic devices in the
1960's,
and
"high temperature" superconductivity in
the 1980's. The invention of
X-ray
crystallography
in the early 1900s by
William Lawrence Bragg enabled
further
innovation.
Although some inorganic
species can be obtained in pure
form from nature,
most
are synthesized in chemical
plants and in the
laboratory.
There
is much chemical ingenuity in
the synthesis of solid
materials. While
tailor-
making
of materials of the desired
structure and properties remains
the main goal of
solid
state
chemistry and material science, it is
not always possible. However, a
rational
approach
to the synthesis of solids
may be evolved which
requires an understanding of
the
principles of crystal chemistry, and of
thermodynamics, phase equilibria and
reaction
kinetics.
Synthetic
Strategies
Various
types of chemical reactions
have been used for
the synthesis of solid
materials.
Some
of common reactions employed
for the synthesis inorganic
solids are listed
below:
16.6
Some
Conceptual Developments in Synthesis in
Chemistry
(1)
decomposition
B(s) + C(g)
A(s)
(2)
combination
C(s)
A(s) +
B(g)
(3)
metathetic [combining (1) and
(2) above]
C(s)
+
D(g)
A(s) +
B(g)
(4)
addition
C(s)
A(s) +
B(s)
C(s)
A(s) +
B(l)
C(s)
A(g) +
B(g)
(5)
exchange
AY(s)
+
BX(s)
AX(s) +
BY(s)
AY(s)
+
BX(g)
AX(s) +
BY(g)
More
complex reactions involving
more than one type of
reaction are also
commonly
employed
in solid state synthesis.
For example, in the
preparation of complex oxides, it
is
common
to carry out thermal
decomposition of a compound followed by
oxidation (in air
or
O2 )
essentially in one step:
CaMnO3(s) + 2CO2(g)
2Ca0.5Mn0.5CO(s)
+ 1/2 O2(g)
O2
MgO(s)
+ Cr2O3(s)
MgCr2O4(s)
Specific
reagents and reaction conditions
are employed to carry out
various processes
such
as reduction, oxidation and halogenation
in the synthesis of
solids.
Synthetic
Strategies in Chemistry
16.7
Reduction
of oxides, for instance, may
be carried out
in
an atmosphere of (flowing) pure or dilute
hydrogen or CO.
�
by
heating oxides in argon or
nitrogen for the purpose of
lowering the oxygen
�
content
by
application of vacuum at an appropriate
temperature (vacuum annealing
or
�
decomposition
at low pressures)
The
obvious means of reducing
solid compounds by hydrogen is
employed not only
for
reducing
oxides, but also halides and
other compounds. Thermal
decomposition of metal
halides
also yields lower
halides:
2MO(s)
+ H2O(g)
M2O3(s) + H2(g)
(eg.
M = Fe)
ABO2.5(s)
+ 1/2H2O(g)
ABO3(s) + H2(g)
(e.g.
LaCoO3)
MCl2(s) + HCl(g)
MCl3(s) + H2(g)
(eg.
M = Fe)
heat
(eg.
M = Fe)
MX2 +
1/2X2
MX3
Reduction
of oxides and halides can also be
accomplished by reacting with
elemental
carbon
or with a metal.
3MCl2 (e.g. M = Nd, Fe)
2MCl3 +
M
3MCl3(s) + M'Cl3(g)
3MCl4 +
M'(s)
(e.g.
M = Hf, M' = Al)
M2O3
+ 3M
5MO
(e.g.
M = Nb)
Inorganic
Synthetic Methods
Some
Conceptual Developments in Synthesis in
Chemistry
16.8
Inorganic
synthetic methods can be classified
roughly according to the
volatility or
solubility
of the component reactants. Soluble
inorganic compounds are
prepared using
the
methods of organic
synthesis.
Techniques
employed in Inorganic
synthesis
Oven
techniques: For
thermally robust materials,
high temperature methods are
often
employed.
For example, bulk solids
are prepared using tubular
furnaces, which allow
reactions
to be conducted up to ca. 1100
�C. Special equipment e.g. ovens
consisting of a
tantalum
tube through which an
electric current is passed, can be
used for even
higher
temperatures
up to 2000 �C.
Melt
methods: If
volatile reactants are involved,
the reactants are often put
in an
ampoule
that is evacuated and then sealed.
The sealed ampoule is then
put in an oven and
given
a certain heat treatment to melt
the reactants together and then
later anneal the
solidified
melt.
Solution
methods: Solvents
are used to prepare solids
by precipitation or by
evaporation.
At
times the solvent is used
hydrothermally, i.e. under
pressure at temperatures higher
than
the normal boiling point. A
variation on this theme is
the use of flux methods,
where
a
salt of relatively low
melting point is added to
the mixture to act as a high
temperature
solvent
in which the desired
reaction can take place.
Gas
reactions: Many
solids react readily with
reactive gases like
chlorine, iodine,
oxygen
etc. Others form adducts
with gases like CO or
ethylene. Such reactions are
often
carried
out in a tube that is open
ended on both sides and
through which the gas is
passed
A
special case of a gas reaction is a
chemical transport reaction
which entails the
reversible
conversion of nonvolatile chemical
compounds into volatile
derivatives. This
method
may also be used as a process
for purification and crystallization of
non-volatile
solids.
These are often carried out
in a sealed ampoule to which a
small amount of a
transport
agent, e.g. iodine is added.
The ampoule is then placed in a
zone oven. This is
essentially
two tube ovens attached to
each other which allows a
temperature gradient to
be
imposed. The volatile derivative
migrates through a sealed, evacuated
glass tube
heated
in a tubular furnace. Elsewhere in
the tube where the
temperature is held at a
different
value, the volatile
derivative reverts to the
parent solid and the
transport agent is
released.
The transport agent is thus
catalytic. The technique
requires that the two
ends of
Synthetic
Strategies in Chemistry
16.9
tube
be maintained at different temperatures.
Such a method can be used to
obtain the
product
in the form of single
crystals suitable for
structure determination by
X-ray
diffraction.
Air
and moisture sensitive
materials:
Many solids are hygroscopic
and/or oxygen
sensitive.
Many halides e.g. are very
'thirsty' and can only be studied in
their anhydrous
form
if they are handled in a
glove box filled with
dry (and/or oxygen-free)
gas, usually
nitrogen.
Synthetic
Methods
A
large variety of inorganic
solid materials has been
prepared in recent years by
traditional
ceramic method, which
involves mixing and grinding
powders of the
constituent
oxides, carbonates and such
compounds and heating them at
high
temperatures
with intermediate grinding
when necessary. The low-temperature
chemical
routes,
however, are of greater interest so as to
have better control of the
structure,
stoichiometry
and phase purity. Noteworthy
chemical methods of synthesis
include the
precursor
method, coprecipitation, combustion
method, the gel method,
topochemical
methods
and high-pressure methods. Some of
the methods are outlined
below.
1.
Ceramic Method
The
most common method of
preparing solid materials is by
reaction of the
component
materials
in the solid state at
elevated temperatures. Several
oxides, sulphides,
phosphides,
have been prepared by this
method. Knowledge of the
phase diagram is
generally
helpful in fixing the
desired composition and conditions
for synthesis.
Platinum,
silica and alumina containers
are generally used for
the synthesis of
metal
oxides,
while graphite containers
are employed for sulphides
and other chalcogenides as
well
as pnictides. If one of the constituents
is volatile or sensitive to the
atmosphere, the
reaction
is carried out in sealed evacuated
capsules. Most ceramic preparations
require
relatively
high temperatures which are
generally attained by resistance
heating. Electric
arc
and skull techniques give temperatures up
to 3300 K while high- power
CO2 lasers
give
temperatures up to 4300 K.
The
ceramic method suffers from
several disadvantages. They are:
1.
When no melt is formed
during the reaction, the
entire reaction has to occur
in
the
solid state, initially by a
phase boundary reaction at
the points of contact
between the
Some
Conceptual Developments in Synthesis in
Chemistry
16.10
components
and later by diffusion of the
constituents through the
product phase. As the
reaction
progresses, diffusion paths become
increasingly longer and the
reaction rate
slower.
The product interface
between the reacting
particles acts as a barrier.
The reaction
can
be speeded up to some extent by
intermittent grinding between
heating cycles.
2.
There is no simple way of
monitoring the progress of
the reaction in the
ceramic
method. It is only by trial and
error (by carrying out
X-ray diffraction and
other
measurements
periodically) that one decides on
appropriate conditions that lead
to
completion
of the reaction. Because of
this difficulty, one frequently
ends up with
mixtures
of reactants and products.
3.
Separation of the desired product
from these mixtures is
generally difficult, if
not
impossible.
4.
It is sometimes difficult to obtain a
compositionally homogeneous product
by
the
ceramic technique, even when
the reaction proceeds almost
to completion.
In
spite of such limitations, ceramic
techniques have been widely
used for the
synthesis
of solid materials. Mention
must be made, among others,
of the use of this
technique
for the synthesis of rare
earth mono-chalcogenides such as
SmS and SmSe. The
method
involves heating the
elements, first at lower temperatures
(870-1170 K) in
evacuated
silica tubes; the contents
are then homogenized, sealed
in tantalum tubes and
heated
to around 2300 K.
Various
modifications of the ceramic
technique have been employed
to overcome
some
of the limitations. One of
these relates to decreasing the diffusion
path lengths. In a
polycrystalline
mixture of reactants, the individual
particles are approximately 10 m
in
size,
representing diffusion distances of
roughly 10,000 unit cells. By
using freeze-
drying,
spray-drying, coprecipitation, and
sol-gel and other techniques, it is
possible to
reduce
the particle size to a few
hundred �ngstr�ms and thus
effect a more
intimate
mixing
of the reactants.
In
spray-drying, suitable constituents
dissolved in a solvent are sprayed in
the form of
fine
droplets into a hot chamber.
The solvent evaporates
instantaneously, leaving
behind
an
intimate mixture of reactants, which on
heating at elevated temperatures gives
the
product.
Synthetic
Strategies in Chemistry
16.11
In
freeze-drying, reactants in a common
solvent are frozen by
immersing in liquid
nitrogen
and the solvent is removed at
low pressures.
In
coprecipitation, the required
metal cations taken as
soluble salts (e.g. nitrates)
are
coprecipitated
from a common medium,
usually as hydroxides, carbonates,
oxalates,
formats
or citrates. In actual practice, one
takes oxides or carbonates of
the relevant
metals,
digests them with an acid
(usually HNO3)
and then the precipitating reagent
is
added.
After filtering the
precipitate and drying, it is heated to
the required temperature
in
a
desired atmosphere to produce the
final product. For example,
tetraethylammonium
oxalate
has been used to prepare
superconducting YBa2Cu408.
The decomposition
temperatures
of the precipitates are generally
lower than the temperatures
employed in
the
ceramic method.
2.
Combustion Method
Combustion
synthesis or self-propagating
high-temperature synthesis makes
use of a
highly
exothermic reaction between
the reactants to produce a flame due to
spontaneous
combustion
which then yields the
desired product or its
precursor in finely divided
form.
Borides,
carbides, oxides, chalcogenides and other
metal derivatives have been
prepared
by
this method. In order for
combustion to occur, one has to ensure
that the initial
mixture
of reactants is highly dispersed and has
high chemical energy. For
example, one
may
add a fuel and an oxidizer
when preparing oxides by the
combustion method,
both
these
additives being removed
during combustion to yield
only the product or
its
precursor.
Thus, one can take a mixture of nitrates
(oxidizer) of the desired
metals along
with
a fuel (e.g. hydrazine,
glycine or urea) in solution, evaporate
the solution to
dryness
and
heat the resulting solid to
around 423 K to obtain spontaneous
combustion, yielding
an
oxidic product in fine
particulate form. Even if
the desired product is not
formed
immediately
after combustion, the fine
particulate nature of the
product facilitates
its
formation
on further heating. In order to
carry out combustion
synthesis, the
powdered
mixture
of reactants (0.1-100 m particle size) is
generally placed in an appropriate
gas
medium
which favours an exothermic
reaction on ignition. The
combustion temperature
is
anywhere between 1500 and 3500 K,
depending on the reaction.
The advantages of
this
method
are that
Some
Conceptual Developments in Synthesis in
Chemistry
16.12
reaction
times are very short since
the desired product results
soon after the
�
combustion.
a
gas medium is not always
necessary.
�
This
is so in the synthesis of borides,
silicides and carbides where
the elements are
quite
stable
at high temperatures. Combustion in a
nitrogen atmosphere yields nitrides.
Nitrides
of
various metals have been
prepared in this manner.
Azides have been used as
sources of
nitrogen.
The following are some
typical combustion
reactions:
MoSi2 +
7MgO
MoO3 +
2SiO2
+
7Mg
WC
+ Al2O3
WO3 + C +
2Al
TiB2 +
5MgO
TiO2 +
B2O3
+
5Mg
N2
N2
TaN
Ta2N
Ta
after
burning
Most
of the ternary or quaternary
oxides can also be prepared by this
method. All the
superconducting
cuprates have been prepared by
this method, although the
resulting
products
in fine particulate form
have to be heated to an appropriate high
temperature in a
desired
atmosphere to obtain the final
cuprate. Table 16.1 lists
typical materials
prepared
by
the combustion
method.

Synthetic
Strategies in Chemistry
16.13
Table
1. Typical materials prepared by
the Combustion method
Oxides
BaTiO3,
LiNbO3,
PbMoO4
Carbides
TiC,
Mo2C,
NbC
Borides
TiB2,
CrB2,
MoB2,
FeB
MoSi2,
TiSi2,
ZrSi2
Silicides
Phosphides
NbP,
MnP, TiP
WS2, MoS2, MoSe2,
TaS2
Chalcogenides
Hydrides
TiH2, NdH2
3.
Precursor Method
Synthesis
of complex oxides by the
decomposition of compound precursors
has been
known
for some time. For
example, thermal decomposition of
LaCo(CN)6.5H20
and
LaFe(CN)6.6H20 in air
readily yields LaCoO3 and
LaFeO3
respectively.
BaTiO3
can be
prepared
by the thermal decomposition of
Ba[TiO(C204)2],
while LiCrO2
can be
prepared
from
Li[Cr(C2O4)2(H20)2].
Ferrite spinels of the
general formula MFe204
(M-Mg, Mn,
Ni,
Co)
are prepared by the thermal
decomposition of acetate precursors of
the type
M3Fe6(CH3COO)1703OH.12C5H5N.
Chromites of type MCr204
are
obtained by the
decomposition
of (NH4)2M(CrO4)2.6H20. Carbonates of
metals such as
calcium,
magnesium,
manganese, iron, cobalt,
zinc and cadmium are all
iso-structural, possessing
the
calcite structure. We can therefore
prepare a large number of carbonate
solid solutions
containing
two or more cations in
different proportions.
Carbonate
solid solutions are ideal
precursors for the synthesis of
monoxide solid
solutions
of rock-salt structure. The
facile formation of rock-salt
oxides by the
decomposition
of carbonates of calcite structure is due
to the close (possible
topotactic)
relationship
between the structures of
calcite and rock-salt. The
monoxide solid
solutions
can
be used as precursors for
preparing spinels and other
complex oxides.
Besides
monoxide
solid solutions, a number of
ternary and quaternary oxides of
novel structures
16.14
Some
Conceptual Developments in Synthesis in
Chemistry
can
be prepared by decomposing carbonate
precursors containing the
different cations in
the
required proportions. A number of
ternary and quaternary metal
oxides of perovskite
and
related structures can be prepared by
employing hydroxide, nitrate and
cyanide solid
solution
precursors as well.
4.
Topochemical Reactions
A
solid state reaction is said
to be topochemically controlled when
the reactivity is
controlled
by the crystal structure
rather than by the chemical
nature of the
constituents.
The
products obtained in many
solid state decompositions
are determined by
topochemical
factors, especially when the
reaction occurs within the
solid without the
separation
of a new phase. In topotactic
solid state reactions, the
atomic arrangement in
the
reactant crystal remains
largely unaffected during
the course of the reaction,
except
for
changes in dimension in one or more
directions. Dehydration of WO3.H20
or
MoO3.H20 to
give WO3
or
MoO3
is one such
reaction. Dehydration of many
other
hydrates
such as VOPO4.2H20
and HMoO2PO4.H20 is also
found to be topotactic.
Intercalation
reactions are generally
topotactic in nature. Decomposition of
V2O5
to
form
V6O13
is a similar
reaction. The reduction of
NiO to nickel metal
proceeds
topochemically.
a)Dehydration
of Mo1_xWxO3.H2O
Mo1_xWxO3 solid solutions can be
prepared by the ceramic
method (by heating
MoO3
and
WO3 in
sealed tubes at around 870 K) or by the
thermal decomposition of
mixed
ammonium
metallates. These methods, however, do
not always yield
monophasic
products
owing to the difference in
volatilities of MoO3 and WO3.
Therefore, it was
sought
to prepare Mo1_xWxO3
by
topochemical dehydration of the
hydrates, the process
being
very gentle. MoO3.H2O and
WO3.H2O
are isostructural and the
solid solutions
between
the two hydrates are
prepared readily by adding a
solution of MoO3 and WO3
in
ammonia
to hot 6M HNO3. The hydrates Mo1_xWxO3.H2O
crystallize in the
same
structure
as MoO3.H2O and
WO3.H2O
with a monoclinic unit cell.
The hydrate solid
solutions
undergo dehydration under
mild conditions (around 500 K)
yielding
Mo1_xWxO3
which
crystallizes in the ReO3-related structure of WO3. The ReO3 structure
of
MoO3 is metastable and is produced only by
topotactic dehydration under
mild
conditions.
This preparation of
ReO3-1ike MoO3 by
mild chemical processing is
Synthetic
Strategies in Chemistry
16.15
significant.
Bulk quantities of MoO3 in
the ReO3
structure
have been prepared by
mild
dehydration
of the hydrate.
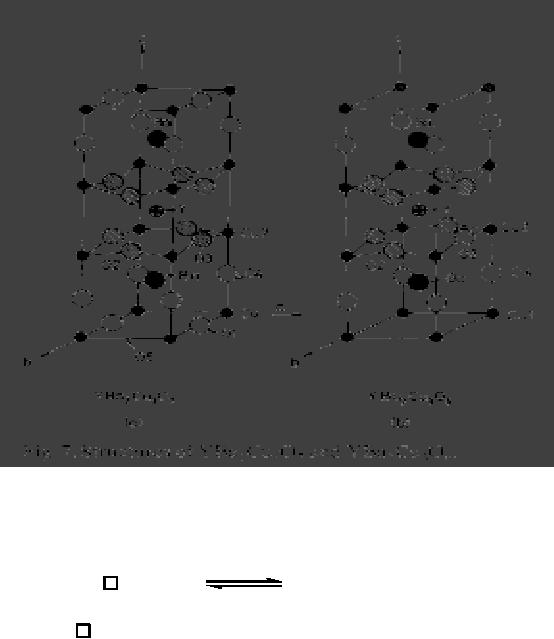
b)Reduction
of perovskite oxides
Reduction
of the high temperature
superconductor YBa2Cu307 to YBa2CuO6 (Fig. 16.7) is
a
topochemical process.
5.
Intercalation Compounds
Intercalation
reactions of solids involve the
insertion of a guest species (ion or
molecule)
into
a solid host lattice without
any major rearrangement of
the solid structure:
x[host]
(guest)x[host]
x(guest)
+
stands
for a vacant lattice
site.
where
Tungsten
and molybdenum bronzes, AxWO3 and AxMoO3
(A=K,
Rb, Cs) are generally
prepared
by reaction of the alkali
metals with the host
oxide. Electrochemical
methods
are
also employed for these
preparations. A novel reaction
that has been employed
to
prepare
bronzes which are otherwise
difficult to obtain involves
the reaction of oxide
host
with
anhydrous alkali
iodides:
16.16
Some
Conceptual Developments in Synthesis in
Chemistry
Mo1-xWxO3
+
y(AI)
→
Ay.Mo1-xWxO3
+
y/2
I2
Atomic
hydrogen has been inserted
into many binary and ternary
oxides. Recently,
iodine
has
been intercalated into the
superconducting cuprate, Bi2CaSr2Cu2O8,
causing an
expansion
of the c-parameter of the unit
cell, without destroying the
superconductivity.
6.
Sol-gel Method
The
sol-gel method is a wet
chemical method and a multi-step
process involving
both
chemical
and physical processes such as
hydrolysis, polymerization, drying
and
densification.
The name "sol-gel" is given to
the process because of the
distinctive
increase
in viscosity which occurs at a
particular point in the
sequence of steps. A sudden
increase
in viscosity is the common
feature in sol-gel processing, indicating
the onset of
gel
formation. In the sol-gel
process, synthesis of inorganic
oxides is achieved
from
inorganic
or organometallic precursors (generally
metal alkoxides). The
important
features
of the sol-gel techniques
are better homogeneity compared
with the traditional
ceramic
method, high purity, lower
processing temperature, more uniform
phase
distribution
in multicomponent systems, better size and
morphological control,
the
possibility
of preparing new crystalline and
non-crystalline materials, and lastly
easy
preparation
of thin films and coatings.
The sol-gel method is widely
used in ceramic
technology.
The important steps in
sol-gel synthesis are:
Hydrolysis.
The process of hydrolysis
may start with a mixture of
a metal alkoxide and
water
in a solvent (usually alcohol) at
the ambient or a slightly
elevated temperature.
Acid
or base catalysts are added
to speed up the
reaction.
Polymerization.
This step involves
condensation of adjacent molecules
wherein H2O
and
ROH
are eliminated and metal
oxide linkages are formed.
Polymeric networks grow
to
colloidal
dimensions in the liquid
(sol) state.
Gelation.
In this step, the polymeric
networks link up to form a
three-dimensional
network
throughout the liquid. The
system becomes somewhat
rigid, characteristic of a
gel.
The solvent as well as water
and alcohol remain inside
the pores of the
gel.
Aggregation
of smaller polymeric units to
the main network continues
progressively on
aging
the gel.
Synthetic
Strategies in Chemistry
16.17
Drying.
Here, water and alcohol are
removed at a moderate temperature (less
than 470
K),
leaving a hydroxylated metal
oxide with residual organic
content. If the objective
is
to
prepare a high surface area
of aerogel powder with low
bulk density, the solvent
is
removed
supercritically.
Dehydration.
This step is carried out
between 670 and 1070 K to drive off
the organic
residues
and chemically bound water,
yielding a metal oxide with
up to 20%-30%
microporosity.
Densification.
Temperatures in excess of 1270 K are
used to form the dense
oxide
product.
Many
complex metal oxides are
prepared by a modified sol-gel
route without
actually
preparing
metal alkoxides. For
example, a transition metal
salt solution is converted
into
a
gel by the addition of an appropriate
organic reagent. In the case of
cuprate
superconductors,
an equimolar proportion of citric acid is
added to the solution of
metal
nitrates,
followed by ethylene diamine
until the solution attains a
pH of 6-6.5. The blue
sol
is concentrated to obtain the
gel. The xerogel is obtained
by heating at approximately
420
K. The xerogel is decomposed at an
appropriate temperature to obtain
the cuprate.
The
sol-gel technique has been
used to prepare sub-micrometre
metal oxide powders
with
a narrow particle size
distribution and unique particle
shapes (e.g. A12O3,
TiO2,
ZrO2,
Fe2O3). Uniform
SiO2
spheres
have been grown from
aqueous solutions of
colloidal
SiO2.
Small metal clusters (e.g.
nickel, copper, gold) have
been prepared by in
situ
chemical reduction of metal
salts. Metal-ceramic composites
(e.g.Ni-AI2O3,
Pt-ZrO2)
can
also be prepared in this manner. By
employing several variants of
the basic sol-gel
technique,
a number of multicomponent oxide systems
have been prepared. Typical
of
these
are: SiO2-B2O3,
SiO2-TiO2,
SiO2-ZrO2,
SiO2-A12O3, ThO2-UO2
. A variety
of
ternary
and still more complex
oxides have been prepared by
this technique.
Different
types
of cuprate superconductors have also
been prepared by this
method. These include
YBa2Cu307,
YBa2Cu408,
Bi2CaSr2Cu208
and Pb2Sr2Ca
1 -xYxCu3O8.
6.
Alkali Flux Method
The
use of strong alkaline media,
either in the form of solid
fluxes or molten (or
aqueous)
solutions,
has enabled the synthesis of
novel oxides. The alkali
flux method stabilizes
higher
oxidation states of the
metal by providing an oxidizing
atmosphere. Alkali
16.18
Some
Conceptual Developments in Synthesis in
Chemistry
carbonate
fluxes have traditionally
been used to prepare
transition metal oxides such
as
LaNiO3. A good
example of an oxide synthesized in a
strongly alkaline medium is
the
pyrochlore,
Pb2(Ru2-xPbx)O7-y where
Pb is in the 4 + state; this oxide is a
bifunctional
electrocatalyst.
The procedure for
preparation involves bubbling
oxygen through a
solution
of lead and rubidium salts in strong KOH at 320 K.
The so-called
alkaline
hypochlorite
method is used in many instances.
For example, La4NiOl0 was prepared by
bubbling
Cl2 gas through a NaOH
solution of lanthanum and nickel
nitrates.
YBa2Cu408
has
been prepared by using a Na2CO3-K2CO3 flux in a flowing
oxygen
atmosphere.
KOH melt has been used to
prepare superconducting Ba1-xKxBiO3.
7.
Electrochemical Method
Electrochemical
methods have been employed
to advantage for the synthesis of
many
solid
materials. Typical of the
materials prepared in this
manner are metal
borides,
carbides,
silicides, oxides and sulphides.
Vanadate spinels of formula
MV2O4
as well
as
tungsten
bronzes have been prepared
by the electrochemical route.
Tungsten bronzes are
obtained
at the cathode when current is
passed through two inert
electrodes immersed in a
molten
solution of the alkali metal
tungstate, A2WO4 and WO3;
oxygen is liberated at
the
anode.
Blue molybdenum bronzes have
been prepared by fused salt
electrolysis. Mono-
sulphides
of uranium, gadolinium, thorium and
other metals are obtained
from a solution
of
the normal valent metal
sulphide and chloride in an NaC1-KCI
eutectic. LaB6,
is
prepared
by taking La2O3 and B2O3
in an
LiBO2-LiF melt and using
gold electrodes.
Crystalline
transition metal phosphides are
prepared from solutions of
oxides with alkali
metal
phosphates and halides. Superconducting
Bal-xKxBiO3
has
been prepared
electrochemically.
Although
the electrochemical method is
old, the processes involved
in the synthesis
of
various solids are not
entirely understood. Generally one
uses solvents whose
decomposition
potentials are high (e.g.
alkali metal phosphates, borates,
fluorides, etc.).
Changes
in melt composition could
cause limitations in certain
instances.
8.
High Pressure Methods
The
use of high pressures for
solid state synthesis has
become increasingly common
in
recent
years. With the development of
high-pressure technology, commercial
equipment
permitting
simultaneous use of both
high-pressure and high-temperature
conditions has
Synthetic
Strategies in Chemistry
16.19
become
available. For the 1-10
kbar pressure range, the
hydrothermal method is
often
employed.
In this method, the reaction
is carried out either in an open or a
closed system.
In
the open system, the solid
is in direct contact with
the reacting gas (F2,
O2 or N2)
which
also serves as a pressure intensifier. A
gold container is generally
used in this type
of
synthesis. This method has
been used for the
synthesis of transition metal
compounds
such
as RhO2,
PtO2
and Na2NiF6
where
the transition metal is in a
higher oxidation
state.
Hydrothermal
high pressure synthesis
under closed system conditions
has been employed
for
the preparation of higher-valence
metal oxides. An internal
oxidant such as KClO3 is
added
to the reactants, which on decomposition
under reaction conditions
provides the
necessary
oxygen pressure. For example,
pyrochlores of palladium(IV) and
platinum(IV),
Ln2M207,
(Ln = rare earth) have been
prepared by this method (970
K, 3 kbar).
(H30)Zr2(PO4)3
and a family
of zero thermal expansion ceramics
(e.g. Ca0.5Ti2P3O12)
have
also been prepared hydrothermally.
Another good example is the
synthesis of
borates
of aluminium, yttrium and such
metals wherein the sesquioxides
are reacted with
boric
acid. Oxyfluorides have been
prepared in HF medium [75].
Zeolites are
generally
prepared
under hydrothermal conditions in
the presence of alkali. The
alkali, the silica
component
and the source of aluminium are
mixed in appropriate proportions and
heated.
The
reactant mixture forms a
hydrous gel which is then
allowed to crystallize
under
pressure
for several hours to several
weeks between 330 and 470 K. In a
typical
synthesis,
AI2O3.3H20
dissolved in concentrated NaOH
solution (20 N) is mixed
with a
1
N
solution
of
Na2SiO3.9H20
to
obtain
a
gel
(of
composition
2.1Na2O.Al2O3.2.1SiO2.60H2O )
which is then crystallized to
give zeolite A. The Na2O-
SiO2-Al2O3-H2O
system yields a large number
of materials with the
zeolitic framework.
Under
alkaline conditions, aluminium is present
as Al(OH)4 anions. The OH-
ions act as
a
mineralizing catalyst while
the cations present in the reactant
mixture determine the
kinds
of zeolite formed.
Besides
water, some inorganic salts
are also encapsulated in some zeolites.
Several
zeolite
structures are found in the
K2O-SiO2-Al2O3-H2O
system as well. Li2O,
however,
does
not give rise to many
microporous materials. Group IIA
cations yield several
zeolitic
products.
16.20
Some
Conceptual Developments in Synthesis in
Chemistry
Zeolitization
in the presence of organic
bases is useful for
synthesizing silica-rich
zeolites.
Silicalite with a tetrahedral
framework enclosing a three-dimensional
system of
channels
(defined by 10 rings wide
enough to absorb molecules up to
0.6 nm in diameter)
has
been synthesized by the
reaction of tetrapropylammonium (TPA)
hydroxide and a
reactive
form of silica between 370 and 470 K.
The precursor crystals have
the
composition
(TPA)20.48SiO2.H20 and the
organic cation is removed by
chemical reaction
or
thermal decomposition to yield
microporous silicalite which
may be considered to be a
new
polymorph of SiO2.
The clathrasil (silica
analogue of a gas hydrate),
dodecasil-1H, is
prepared
from an aqueous solution of
tetramethoxysilane and N(CH3)4OH;
after the
addition
of aminoadamantane, the solution is treated
hydrothermally under nitrogen
for
four
days at 470 K. The use of template
cations has enabled the
synthesis of a variety of
zeolite
materials. Cations such as
(NMe4)+
fit snugly into the
cages (e.g. sodalite cages
of
sodalite
and SAPO or gmelinite cages of
zeolites omega). Neutral organic amines
have
also
been used (e.g. in the
synthesis of ZSM-5). Many
new microporous
materials,
including
those based on AlPO4 (analogue of SiO2),
gallosilicates and
aluminogerminates
(analogues
of aluminosilicates), have been prepared.
AlPO4-based
materials are
prepared
by
the crystallization of gels formed by
adding an organic template to a
mixture of active
alumina,
H3PO4
and water at a
pH of 5-8 around 470 K. Pressures in
the range 10-150
kbar
are commonly used for
solid-state synthesis. In the
piston-cylinder apparatus
consisting
of a tungsten carbide chamber and a
piston assembly, the sample is
contained
in
a suitable metal capsule surrounded by a
pressure-transducer (pyrophyllite). Pressure
is
generated
by moving the piston through
the blind hole in the
cylinder. A microfurnace
made
of graphite or molybdenum is incorporated
in the design. Pressures up to 50
kbar
and
temperatures up to 1800 K are readily
reached in a volume of 0.1
cm3 using this
design.
In the anvil apparatus, first
designed by Bridgman, the sample is
subjected to
pressure
by simply squeezing it between
two opposed anvils. Although
pressures of
around
200 kbar and temperatures up to 1300 K are
reached in this technique, it is
not
popular
for solid-state synthesis since
only milligram quantities can be
handled. An
extension
of the opposed anvil
principle is the tetrahedral
anvil design, where
four
massively
supported anvils disposed
tetrahedrally ram towards
the centre where
the
sample
is located in a pyrophyllite medium
together with a heating
arrangement. The
Synthetic
Strategies in Chemistry
16.21
multi-anvil
design has been extended to
cubic geometry, where six
anvils act on the
faces
of
a pyrophyllite cube located at the
centre. The belt apparatus
provides the best
high-
pressure-high-temperature
combination for solid-state
synthesis. This apparatus,
which
was
used for the synthesis of
diamonds some years ago is a
combination of the
piston-
cylinder
and the opposed anvil designs.
The apparatus consists of two
conical pistons
made
of tungsten carbide, which ram
through a specially shaped
chamber from opposite
directions.
The chamber and pistons are
laterally supported by several steel
rings making
it
possible routinely to reach fairly high
pressures (around 150 kbar) and
high
temperatures
(approximately 2300 K). In the
belt apparatus, the sample is contained
in a
noble
metal capsule (a BN or MgO container is
used for chalcogenides) and
surrounded
by
pyrophyllite and a graphite sleeve, the
latter serving as an internal
heater. In a typical
high-pressure
run, the sample is loaded, the
pressure raised to the
desired value and
then
the
temperature increased. After holding
the pressure for about 30
min, the sample is
quenched
(400 Ks-1) while still under
pressure. The pressure is released
after the sample
has
cooled at room
temperature.
High-pressure
methods have been used
for the synthesis of several
materials that
cannot
possibly be made otherwise. In
general, the formation of a
new compound from
its
components
requires that the new
composition have a lower
free energy than the
sum of
the
free energies of the
components. Pressure can aid in the
lowering of free energy
in
different
ways.
(a)Pressure
delocalizes outer d electrons in
transition-metal compounds by increasing
the
magnitude
of coupling between the d
electrons on neighbouring cations,
thereby lowering
the
free energy. A typical
example is the synthesis of
ACrO3 (A = Ca, Sr, Pb)
perovskites
and
CrO2.
(b)
Pressure stabilizes higher-valence
states of transition metals,
thus promoting the
formation
of a new phase. For example,
in the Ca-Fe-O system only
CaFeO2.5 (brown-
millerite)
is stable under ambient pressures.
Under high oxygen pressures,
iron is
oxidized
to the 4+ state and hence CaFeO3 with the perovskite
structure is formed.
(c)
Pressure can suppress the
ferroelectric displacement of cations,
thereby adding the
synthesis
of new phases. The synthesis
of A~MoO3 bronzes, for
example, requires
16.22
Some
Conceptual Developments in Synthesis in
Chemistry
populating
the empty d orbitals centred
on molybdenum; at ambient pressures,
MoO3 is
stabilized
by a ferroelectric distortion of
MoO6 octahedra up to the melting
point.
(d)
Pressure alters site-preference
energies of cations, and facilitates
the formation of new
phases.
For example, it is not possible to
synthesize A2+Mn4+O3
(A = Mg,
Co, Zn)
ilmenites
because of the strong
tetrahedral site preference of the
divalent cations. One
therefore
obtains a mixture of A[AMn]O4(spinel)
+ MnO2(rutile) under
atmospheric
pressure
instead of monophasic AMnO3.
However, the latter is
formed at high
pressures
with
a corundum-type structure in which
both the A and Mn ions are
in octahedral
coordination.
(e)
Pressure can suppress the
6s2 core
polarization in oxides containing
isoelectronic
Tl+,
Pb2+, Bi3+
cations.
For example, perovskite-type
PbSnO3 cannot be made at
atmospheric
pressure because the mixture
of PbO + SnO2 is more stable than
the
perovskite.
Thus,
solid-state reactions are
generally slow under
ordinary pressures even when
the
product
is thermodynamically stable. Pressure has
a marked effect on the
kinetics of the
reaction,
reducing the reaction times
considerably, and at the same
time giving more
homogeneous
and crystalline products. For
instance, LnFeO3,
LnRhO3
and
LnNiO3
(Ln
=
rare
earth) are prepared in a
matter of hours under
high-pressure-high-temperatue
conditions,
whereas at ambient pressure the
reactions require several days
(LnFeO3 and
LnRhO3) or
they do not occur at all
(LnNiO3
). Thus
LnFeO3
is formed in
30 min at 50
kbar.
In
several (AX)(ABX3)n series of compounds, the
end members ABX3 and A2BX4,
having
the perovskite and K2NiF4
structures
respectively, are formed at
atmospheric
pressures,
but not the intermediate
phases such as A3B2X7
and A4B3X10.
Pressure
facilitates
the synthesis of such
solids. Sr3Ru2O7
and Sr4Ru3O10
are
formed in 15 min at
20
kbar and 1300 K.
High-pressure
methods
have
been
employed
in
the
synthesis
of
novel
superconducting
cuprates. A rudimentary example is the
preparation of oxygen-excess
La2CuO4
under
high oxygen pressure. A more
interesting example is the
synthesis of the
next
homologue with two
CuO2 layers. La2Cal- xSrxCu2O6
which had
earlier been found
to
be an insulator was rendered superconducting by
heating it under oxygen
pressure.
Synthetic
Strategies in Chemistry
16.23
YBa2Cu408 was first prepared under
high oxygen pressure, but
this was soon found
unnecessary.
However, superconducting cuprates with
infinite CuO2 layers of the
type
Ca1-xSrxCuO2
or Sr1-xNdxCuO2
can only be
prepared under high
hydrostatic pressures
which
help to give materials with
shorter Cu-O bonds. It should be
noted that Ca1-
xSrxCuO2
prepared
at ambient pressure is
insulating.
9.
The pyrosol Process: a novel
chemical method for
depositing films
Pyrolysis
of sprays is a well known method
for depositing films. Thus,
one can obtain
films
of oxidic materials such as
CoO, ZnO and YBa2Cu307 by the spray pyrolysis
of
solutions
containing salts (e.g. nitrates) of
the cations. A novel
improvement in this
technique
is the pyrosol process
involving the transport and subsequent
pyrolysis of a
spray
generated by an ultrasonic atomizer.
When a high frequency (100
kHz-10 MHz
range)
ultrasonic beam is directed at a
gas-liquid interface, a geyser is formed
and the
height
of the geyser is proportional to the
acoustic intensity. Its
formation is accompanied
by
the generation of a spray,
resulting from the
vibrations at the liquid
surface and
cavitation
at the liquid-gas interface.
The quantity of spray is a
function of the
intensity.
Ultrasonic
atomization is accomplished using an
appropriate transducer made of
PZT
located
at the bottom of the liquid
container. A 500-1000 kHz
transducer is generally
adequate.
The atomized spray which
goes up in a column fixed to
the liquid container
is
deposited
onto a suitable solid substrate and
then heat treated to obtain the
film of the
material
concerned. The flow rate of
the spray is controlled by
the flow rate of air or
any
other
gas. The liquid is heated to
some extent, but its
vaporization should be avoided.
The
source
liquid contains the relevant
cations in the form of salts
dissolved in an organic
solvent.
Organometallic compounds are
often used (e.g. acetates,
alkoxides,
acetylacetonates,
etc.). Proper gas flow is
crucial to obtain satisfactory
conditions for a
good
liquid spray.
The
pyrosol process is in some
way between chemical vapour
deposition and spray
pyrolysis,
but the choice of source
compounds for the pyrosol
process is larger than
that
available
for chemical vapour
deposition. Films of a variety of
materials have been
obtained
by the pyrosol method. The
thickness of the films can be
anywhere between a
few
hundred angstroms to a few
micrometres. Films of superconducting
cuprates such as
16.24
Some
Conceptual Developments in Synthesis in
Chemistry
YBa2Cu3O7 have also been prepared by
the pyrosol process. Epitaxy
has been observed
in
films deposited onto
single-crystal substrates.
10.
Czochralski Process for Semiconductor
Materials
Semiconductors
with predictable, reliable
electronic properties are necessary for
mass
production.
The level of chemical purity
needed is extremely high
because the presence
of
impurities even in very
small proportions can have
large effects on the properties
of
the
material. A high degree of
crystalline perfection is also required,
since faults in
crystal
structure (such as dislocations,
twins, and stacking faults)
interfere with the
semiconducting
properties of the material.
Crystalline faults are a
major cause of
defective
semiconductor devices. The larger
the crystal, the more
difficult it is to achieve
the
necessary perfection. Current
mass production processes
use crystal ingots
between
four
and twelve inches (300 mm)
in diameter which are grown
as cylinders and sliced
into
wafers.
Because
of the required level of
chemical purity and the
perfection of the
crystal
structure
which are needed to make
semiconductor devices, special methods
have been
developed
to produce the initial
semiconductor material. A technique
for achieving high
purity
includes growing the crystal
using the Czochralski
process. An additional step
that
can
be used to further increase
purity is known as zone
refining. In zone refining,
part of
a
solid crystal is melted. The
impurities tend to concentrate in
the melted region,
while
the
desired material recrystalizes
leaving the solid material
more pure and with
fewer
crystalline
faults. In manufacturing semiconductor
devices involving heterojunctions
between
different semiconductor materials,
the lattice constant, which
is the length of the
repeating
element of the crystal
structure, is important for
determining the
compatibility
of
materials.
The
Czochralski process is a method of
crystal growth used to
obtain single
crystals
of
semiconductors (e.g. silicon,
germanium and gallium arsenide), metals
(e.g. palladium,
platinum,
silver, gold), salts and some
man-made (or lab) gemstones.
The most important
application
may be the growth of large
cylindrical ingots, or boules, of
single crystal
silicon.
Other semiconductors, such as
gallium arsenide, can also be grown by
this
method,
although lower defect densities in
this case can be obtained
using variants of the
Bridgeman
technique.
Synthetic
Strategies in Chemistry
16.25
High-purity,
semiconductor-grade silicon (only a
few parts per million of impurities)
is
melted
down in a crucible , which is
usually made of quartz.
Dopant impurity atoms
such
as
boron or phosphorus can be added to
the molten intrinsic silicon
in precise amounts in
order
to dope the silicon, thus
changing it into n-type or
p-type extrinsic silicon.
This
influences
the electrical conductivity of
the silicon. A seed crystal,
mounted on a rod, is
dipped
into the molten silicon.
The seed crystal's rod is
pulled upwards and rotated at
the
same
time. By precisely controlling
the temperature gradients, rate of
pulling and speed
of
rotation, it is possible to extract a
large, single-crystal, cylindrical
ingot from the
melt.
Occurrence
of unwanted instabilities in the
melt can be avoided by
investigating and
visualizing
the temperature and velocity
fields during the crystal
growth process [1].
This
process is normally performed in an
inert atmosphere, such as argon, and in
an inert
chamber,
such as quartz.
Size
of Crystals:
While the largest silicon
ingots produced today are
400 mm in diameter
and
1 to 2 metres in length, 200 mm and 300 mm diameter
crystals are standard
industrial
processes.
Thin silicon wafers are
cut from these ingots
(typically about 0.2 - 0.75
mm
thick)
and can be polished to a very high
flatness for making
integrated circuits, or
textured
for making solar
cells.
Impurity
Incorporation:
When silicon is grown by the
Czochralski method the melt
is
contained
in a silica (quartz) crucible.
During growth the walls of
the crucible dissolve
into
the melt and Czochralski
silicon therefore contains
oxygen impurities with a
typical
concentration
of 1018 cm
-3
.
Perhaps surprisingly, oxygen
impurities can have
beneficial
effects.
Carefully chosen annealing conditions can
allow the formation of
oxygen
precipitates.
These have the effect of
trapping unwanted transition
metal impurities in a
process
known as gettering. Additionally,
oxygen impurities can improve
the mechanical
strength
of silicon wafers by immobilising
any dislocations which may
be introduced
during
device processing. It has experimentally
been proved in the 1990s
that the high
oxygen
concentration is also beneficial for
radiation hardness of silicon
particle detectors
used
in harsh radiation environment (
eg. CERN's LHC/S-LHC
projects) [2, 3, 4].
Therefore,
radiation detectors made of Czochralski-
and Magnetic Czochralski-silicon
are
considered
to be promising candidates for
many future high-energy
physics
experiments.[5][6] However,
oxygen impurities can react with
boron in an illuminated
16.26
Some
Conceptual Developments in Synthesis in
Chemistry
environment,
such as experienced by solar cells.
This results in the
formation of an
electrically
active boronoxygen complex
that detracts from cell
performance. Module
output
drops by approximately 3% during the
first few hours of light
exposure [7].
The
Bridgman
technique is
a method of growing single
crystal ingots or boules.
The
method
involves heating polycrystalline
material in a container above its
melting point
and
slowly cooling it from one end
where a seed crystal is
located. Single crystal
material
is
progressively formed along
the length of the container.
The process can be carried
out
in
a horizontal or vertical geometry. It is
a popular method of producing
certain
semiconductor
crystals, such as gallium
arsenide where the
Czochralski process is
more
difficult.
Purification
process: Zone melting is
a method of separation by melting in
which a
molten
zone traverses a long ingot of
impure metal or
chemical.
Zone
refining: In
zone refining, solutes are
segregated at one end of the ingot in
order to
purify
the remainder. The molten
region melts impure solid at
its forward edge and
leaves
a
wake of purer material
solidified behind it as it moves
through the ingot. The
impurities
concentrate
in the melt, and are moved
to one end of the ingot. Zone
refining was
developed
in Bell Telephone Laboratories as a
method to prepare high
purity materials
for
manufacturing transistors. Its
early use was on germanium
for this purpose, but it
can
be
extended to virtually any
solute-solvent system having an
appreciable concentration
difference
between solid and liquid
phases at equilibrium. This
process is also known as
the
Float
zone process,
particularly in semiconductor materials
processing.
Zone
leveling : Zone
melting is also used to concentrate
the impurities for
analytical or
other
purposes. In zone leveling, the
objective is to distribute solute
evenly throughout
the
purified material, which may
be sought in the form of a
single crystal. For
example,
in
the preparation of a transistor or diode
semiconductor, an ingot of germanium is
first
purified
by zone refining. Then a
small amount of antimony is placed in
the molten zone,
which
is passed through the pure
germanium. With the proper
choice of rate of heating
and
other variables, the
antimony can be spread evenly
through the germanium.
This
technique
is also used for the
preparation of silicon for
use in computer
chips.
Heaters:
A
variety of heaters can be used
for zone melting, with
their most important
characteristic
being the ability to form
short molten zones that move
slowly and
Synthetic
Strategies in Chemistry
16.27
uniformly
through the ingot. Induction
coils, ring-wound resistance heaters, or
gas flames
are
common methods. Another
method is to pass an electric
current directly through
the
ingot
while it is in a magnetic field,
with the resulting
magnetomotive force carefully
set
to
be just equal to the weight
in order to hold the liquid
suspended. Zone melting can
be
done
as a batch process, or it can be done
continuously, with fresh
impure material being
continually
added at one end and purer material
being removed from the
other, with
impure
zone melt being removed at
whatever rate is dictated by the
impurity of the feed
stock.
Zone
Remelting: Another related
process is zone remelting, in
which two solutes
are
distributed
through a pure metal. This
is important in the manufacture of
semiconductors,
where
two solutes of opposite conductivity
type are used. For
example, in germanium,
pentavalent
elements of group V such as
antimony and arsenic produce negative
(n-type)
conduction
and the trivalent elements of
group III such as aluminium and
boron produce
positive
(p-type) conduction. By melting a
portion of such an ingot and
slowly refreezing
it,
solutes in the molten region
become distributed to form
the desired n-p and
p-n
junctions.
Biocatalysis
Pharmaceutical
industry has been a rapidly
growing field. About 54% of
drug molecules
are
chiral, and resolution remains an
important and cost-effective approach to
chiral
molecules.
In order to achieve a typical
enantiomeric purity of 99.5%,
resolution is often
accomplished
at the price of restricting
the overall maximum yield of
a process to 50% at
most.
While metal catalyzed reactions
have become prevalent in
recent years, their use
in
manufacturing
requires great effort to identify
appropriate catalysts, solvents and
reaction
conditions.
Reproducibility and robustness can remain a
problem, especially with
regard
to
sensitivity to substrate quality and
small variation in reaction parameters.
There is a
clear
need for new methodologies
to produce chiral molecules.
Enzymes are highly
efficient
with excellent regioselectivity and
stereoselectivity. By conducting
reactions in
water
under ambient reaction
conditions, both the use of
organic solvents and
energy
input
are minimized. Furthermore, one can
design processes with high
energy efficiency
and
safe chemistry by conducting
reactions at ambient temperature
under ambient
16.28
Some
Conceptual Developments in Synthesis in
Chemistry
atmosphere,
and atom economy can be increased by
avoiding extensive protection
and
deprotection
sequences.
Biocatalysis
is emerging as one of the greenest
technologies as a result of recent
advances
in
genomics, proteomics and pathway
engineering (large scale DNA
sequencing,
structural
biology, protein expression,
high throughput screening,
directed enzyme
evolution
and metabolic engineering). Application
of the twelve principles of
green
chemistry
can deliver higher efficiency and reduce
the environmental burden
during
chemical
synthesis.
As
the green chemistry movement gains
momentum, fresh opportunities of
research
and
development have been opened
up to improve the efficiency of
chemical processes
while
simultaneously reducing production
costs. The attention is
focused on reducing
the
use
and generation of large quantities of
hazardous substances which
frequently
accompanies
the synthesis of a pharmaceutical
agent, at each of the
numerous steps, each
of
which involve feedstocks, reagents,
solvents, and separation. The following
case
studies
depict the developments and
improvements in the synthetic
strategies of various
chemicals.
CASE
STUDY 1: Production of LY300164
(Talampanol) :
An example of the
reduction
of hazardous materials is the
use of a chemoenzymatic synthesis
for the
production
of LY300164 (Talampanol) for
treating epilepsy and
neurodegenerative
diseases.
The first generation
synthesis, which starts from
5-allyl-1,3-benzodioxole 1,
suffers
from a low yield of 16%, and
requires the use of a large
amount of organic
solvents
and chromium oxide, a cancer-suspect agent, to
oxidize racemic alcohol 2 to
ketone
3
(Scheme
16.1).
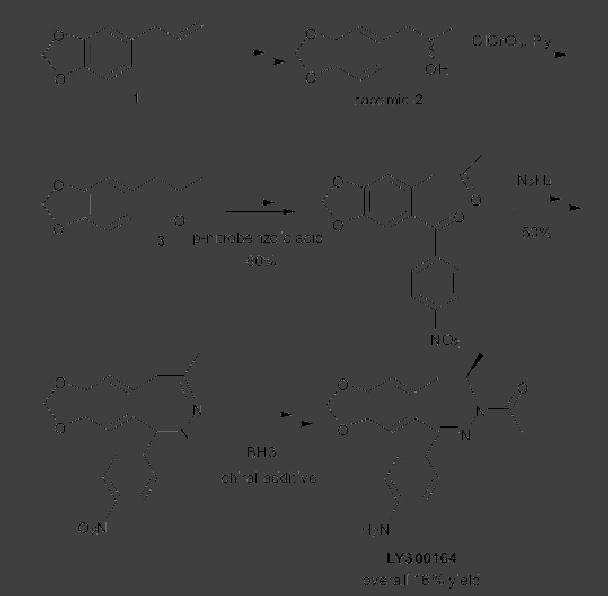
Synthetic
Strategies in Chemistry
16.29
Scheme
16.1.
In
order to improve its
synthesis, a second generation
route was developed involving
a
biocatalytic
ketone reduction of 3
by
Zygosaccharomyces rouxii to give
(S)-2
with
a
yield
of 96% and >99.9% ee (enantiomeric
excess) (Scheme 16.2).
Acid-catalyzed
reaction
with 4-nitrobenzaldehyde led to
1-arylisochroman 4, which
was subsequently
oxidized
to ketal 5
in
the presence of oxygen. The
final product LY300164 was
obtained
after
formation of hydrazone 6, and
cyclization via mesylate 7 to
give 8
followed
by Pd-
catalyzed
nitro group
reduction.
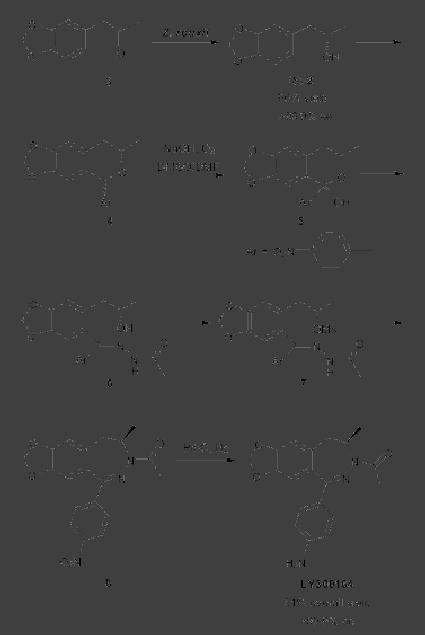
16.30
Some
Conceptual Developments in Synthesis in
Chemistry
Scheme
16.2.
By
using a chemoenzymatic approach, not
only was the overall yield
improved to 51%
from
16% in the old route,
the new synthetic pathway
eliminated the use of
transition
metal
oxidants and a large volume of
organic solvents by judicious
adjustment of
oxidation
states and integration of a
biotransformation. For example,
using the
biocatalytic
process, approximately 340000 L of
solvents and 3000 kg of chromium
waste
were eliminated for the
production of every 1000 kg of
LY300164.
CASE
STUDY 2: Production of Pregabalin
Synthetic
Strategies in Chemistry
16.31
Most
enzymatic catalysis achieves high
regio- and stereoselectivity under
mild
conditions,
allowing new and more
efficient processes to be designed
with significant
advantages
over chemical approaches.
For example, in the first
generation route for
the
production
of pregabalin, chemical resolution of
the racemic γ-amino acid 11,
which was
prepared
from the racemic
cyanodiester 9
(CNDE)
after hydrolysis and
decarboxylation,
took
place at the end of the process (Scheme
3). To obtain a high optical
purity (99.5%)
of
the final API, (S)-mandelic
acid resolution was followed by another
step of
recrystallization
in THF/H2O
with a combined two-step
yield of 2529%. As a result,
the
overall
yield for the route is
only 1821% and over 70%
of all process materials
before
the
resolution step including
nickel were ultimately
turned into wastes. Since
the
undesired
enantiomer could not be
recycled, this modest efficiency
leads to a large
amount
of wastes and excessive reactor capacity
requirements.
To
overcome these issues, a
second generation process
using a fungal lipase was
developed,
where enzymatic resolution
occurred at the first step
and the undesired
enantiomer
13
could be
easily recycled (Scheme 4).
Regio- and stereospecific
hydrolysis
of
9
led to
the mono acid 12
with
45% conversion and >98%
ee. After decarboxylation
of
12
and
phase splitting, mono ester
14
was
telescopically subjected to basic
hydrolysis and
hydrogenation
to give the final product.
Simple operation as a result of
using water as the
solvent
significantly improved process
efficiency. Comparing with
the first generation
synthesis,
not only are a large
amount of (S)-mandelic acid completely
eliminated, the
biocatalytic
process also rendered it unnecessary to
use most organic solvents.
This
stands
in contrast to a typical process
for the production of an
API, where 80% of
all
wastes
are solvents. If spent solvents
are incinerated instead of being
recovered, the life-
cycle
profile and impacts are
considerably increased. Moreover,
recycling of the
undesired
enantiomer 13
doubled
both the yield (40%
vs. <21%) and throughput.
Overall,
it
was projected that at the
peak of manufacturing, the
biocatalytic aqueous process
would
annually
eliminate the usage of over
thousands of metric tons of
rawmaterials including
mandelic
acid, CNDE and nickel, and tens millions
of gallons of alcoholic solvents
and
THF
associated with the classic
resolution route.
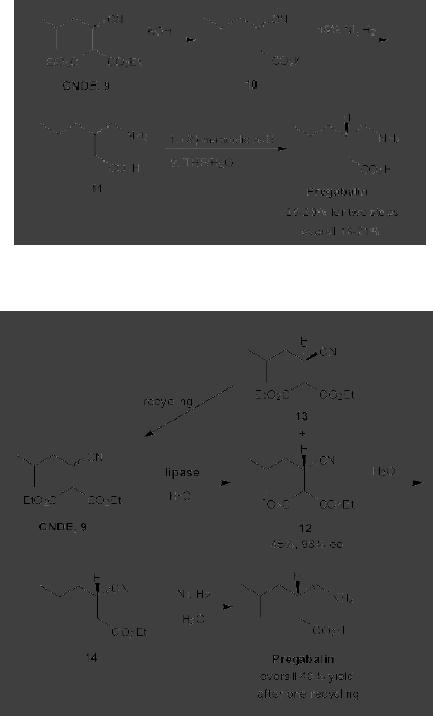
16.32
Some
Conceptual Developments in Synthesis in
Chemistry
Scheme
16.3.
Scheme
16.4.
CASE
STUDY 3: Atorvastatin Calcium
Atorvastatin
calcium is the active
ingredient of Lipitor R, the
first drug with annual
sales
exceeding
$10B. In the current
process, the key chiral
building block in the
synthesis of
atorvastatin
is ethyl (R)-4-cyano-3-hydroxybutyrate (15)
with an annual demand
estimated
to be about �440 000, which was
then converted to atorvastatin
side chain 18
upon
Claisen condensation, borane-chelation
controlled reduction, both
under cryogenic
Synthetic
Strategies in Chemistry
16.33
conditions,
followed by protection of the
two hydroxy groups and
Ni-catalyzed nitrile
group
hydrogenation. The final API
atorvastatin was produced after
PaalKnorr
condensation
of 18
with a
diketone (Scheme 5). A number of
biocatalytic approaches
have
been reported for the
synthesis of (R)-4-cyano-3-hydroxybutyrate
15.
For example,
a
green process has recently
been reported using a
two-enzyme system under
neutral
conditions
in water (eq. 1, Scheme
16.6). In this process, the
first step involves
enantioselective
enzymatic reduction of ethyl
4-chloroacetoacetate to give 19
followed
by
a
biocatalytic cyanation of the
chlorohydrin to produce 15.
Alternatively,
15
was
synthesized from inexpensive
racemic epichlorohydrin
via
nitrilase-catalyzed
desymmetrization of meso-3-hydroxyglutaronitrile
20
(eq. 2,
Scheme
6).
By applying gene site saturation
mutagenesis to improve the
stereoselectivity of the
biocatalyst,
the ee of the desired
product reached 99% under a
3M loading of the
substrate.
The synthesis is short and
utilizes an inexpensive starting
material. Both the
ketoreductase
(eq. 1, Scheme 6) and nitrilase
processes (eq. 2, Scheme 6)
led to
significant
reduction of byproducts, wastes and
organic solvents associated
with existing
chemical
routes. Recently, a more concise approach
to install the atorvastatin
side chain
was
reported by using a microbial
deoxyribose-5-phosphate aldolase (DERA).
This
enzyme
catalyzes the sequential
aldol condensation between one
equivalent of amino
aldehye
21
and
two equivalents of acetaldehyde to form
lactol 22
with
excellent ee (98%)
and
de (97%), which was then
converted to the statin side
chain 18
upon
oxidation,
protection
and esterification. Using this
method, the overall process
to atorvastatin was
shortened
significantly and two cryogenic
steps in the existing
process were
eliminated
(Scheme
7). It is estimated that hundreds of
metric tons of raw materials
and solvents will
be
reduced each year by using
the chemoenzymatic route in
concomitant with
significant
reduction
in energy consumption.
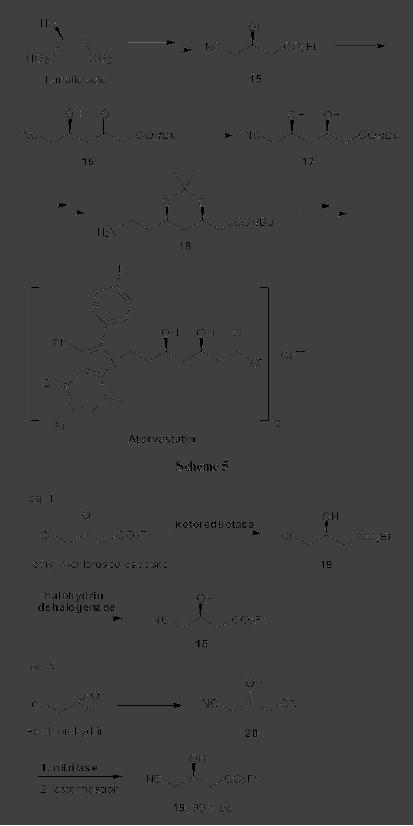
16.34
Some
Conceptual Developments in Synthesis in
Chemistry
Scheme
16.6.

Synthetic
Strategies in Chemistry
16.35
Scheme
16.7.
Rosuvastatin
:
Similar
approaches have been applied
to the synthesis of the side
chain of rosuvastatin,
the
API of Crestor(R) (Scheme 8). By a
combination of activity- and
sequence-based
screening,
a Novel DERA was discovered
from environmental DNA libraries
leading to
an
enzymatic process to obtain
lactol 23
with
volumetric productivity of 720 g L-1 day-1
under
an enzyme loading of 2%
wt/wt.
Scheme
16.8.
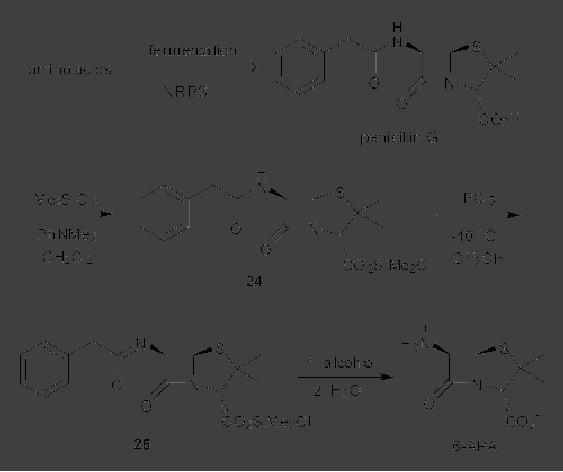
16.36
Some
Conceptual Developments in Synthesis in
Chemistry
CASE
STUDY 4: Industrial Production of
b-lactam Antibiotics :
Biocatalysis
is uniquely suited to the
development of green chemistry routes
for complex
molecules,
which are often labile and
densely functionalized. As a result of
high
selectivity
and mild conditions, enzymatic
catalysis has been applied
to the industrial
production
of b-lactam antibiotics, which
are mostly derived from
6-aminopenicillanic
acid
(6-APA) or 7-aminodesacetoxycephalosporanic acid
(7-ADCA). The annual
world
production
of 6-APA and 7-ADCA were estimated to be
over 8000 and 600 t,
respectively.
Until recently, they were
prepared from penicillin G, a
fermentation product
derived
from non-ribosomal peptide synthase
(NRPS), by chemical deacylation,
where
the
carboxy group of the
penicillin G is first protected by
silylation, followed by
selective
deacylation
via the imidoyl chloride
(25),
and removal of the protecting
group (Scheme
9).
The method uses
stoichiometric amounts of the
silylating agent, and a large amount
of
hazardous
chemicals
and
solvents
such
as
phosphorus
pentachloride
and
dichloromethane.
Scheme
16.9
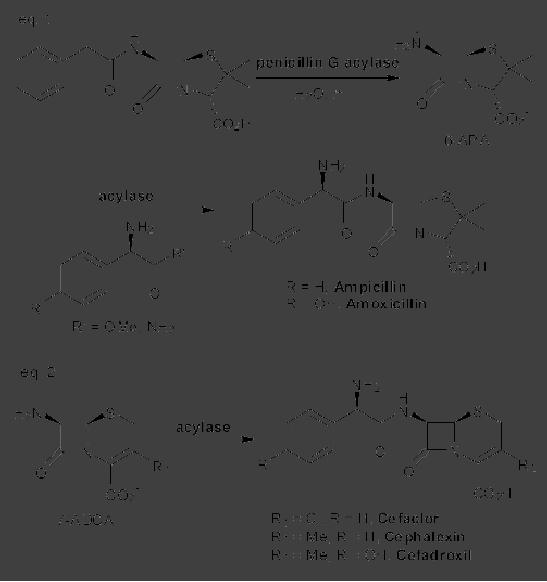
Synthetic
Strategies in Chemistry
16.37
In
contrast, enzymatic deacylation of
penicillinGby penicillin G acylase
was
accomplished
in water at room temperature
requiring no protection and deprotection
of
other
functional groups (Scheme 10).
Moreover, under kinetic
control, an immobilized
penicillin
aclyase was also able to catalyze the
acylation of 6-APA and 7-ADCA
with
either
D-phenylglycine methyl ester
(PGA), D-phenylglycine amides
(PGA) or their
parahydroxylated
analogs to produce a wide range of
semi-synthetic b-lactam
antibiotics
such
as ampicillin, amoxicillin, cefaclor,
cephalexin and cefadroxil (Scheme 16.10).
As a
result
of the biotransformation, raw
material efficiency and E-factor
were significantly
improved
from the first generation of
chemical processes.
Scheme
16.10.
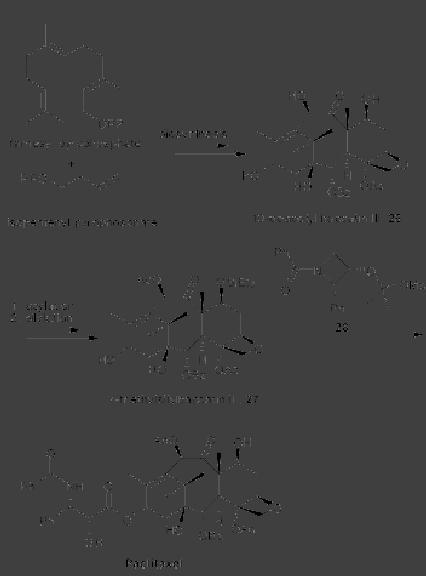
16.38
Some
Conceptual Developments in Synthesis in
Chemistry
CASE
STUDY 5: Paclitaxel (Taxol
R_)
Plant
secondary metabolites have
provided a rich and renewable resource of
natural
products
for drug development.
However, establishing a stable supply of
the active
compounds
from plants is often
difficult. A prominent example is
Paclitaxel (Taxol
R_),
a
complex diterpenoid alkaloid
originally isolated from the
bark of the pacific yew
tree
Taxus
brevifolia with a yield of
0.014%. In addition, isolating
Paclitaxel required
stripping
the bark from the
yew trees, thus killing a
slow growing tree in the
process,
which
takes about 200 years to
mature.
Scheme
16.11
On
the other hand, the
complexity of the Paclitaxel
molecule makes
commercial
production
by chemical synthesis from
simple compounds impractical. As a
result, a
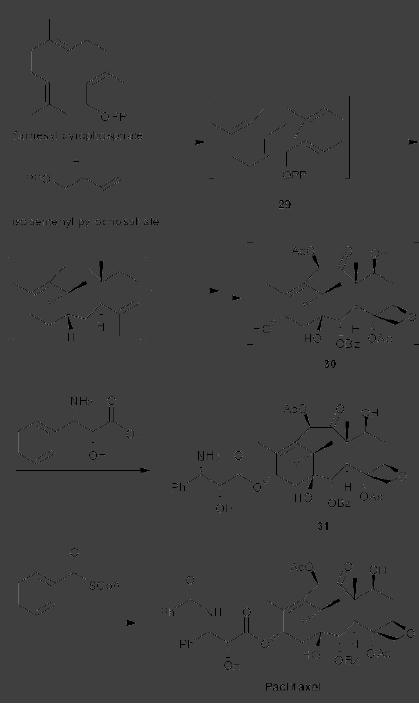
Synthetic
Strategies in Chemistry
16.39
semisynthetic
process
was developed starting from
10-deacetylbaccatin III (Scheme
16.11),
Scheme
16.12.
a
more abundant taxoid
biosynthesized from isoprenyl
diphosphate and farnesyl
diphosphate
in the needles of the
European yew tree Taxus
baccata, which could
be
isolated
with a yield of 0.1% without
harm to the trees. In this
route, 10-deacetylbaccatin
16.40
Some
Conceptual Developments in Synthesis in
Chemistry
III
was first acetylated and silylated.
The resulting 7-triethylsilyl
baccatin III (27)
was
then
coupled to an N-acyl-blactam (28) to
install a phenylisoserine side
chain at the C-
13
position followed by deprotection to
afford Paclitaxel. Overall,
however, the
semisynthetic
process is still complex
requiring eleven chemical
transformations
including
the preparation of 28
and seven
isolations.
Consequently,
an alternative
process
to Paclitaxel was developed using Taxus
cell fermentation and extraction
from
culture
medium, followed by
recrystallization.
Starting
from the two isoprenoid
precursors, isoprenyl diphosphate and
farnesyl
diphosphate
(Scheme 12), geranylgeranyl pyrophosphate
synthetase catalyzes
the
coupling
to give geranylgeranyl pyrophosphate,
29,
which is then cyclized and
then
converted
to baccatin III 30
through a
series of enzymatic
transformationsincluding
hydroxylation,
acylation, oxidation and generation of
the oxetane ring. The
side chain in
31
was
installed by enzymatic transfer of
phenylisoserine. To achieve a high
titer of
Paclitaxel
production, cell cultures
from various species of
Taxus and different
elicitors
to
induce Paclitaxel production
have been examined. For
example, methyl jasmonate
was
able
to enhance Paclitaxel production to 110
mg L-1 every 2 weeks in cell
suspension
culture
of T. media. The plant cell
fermentation process led to an
elimination of the
eleven
chemical transformations and a large
amount of hazardous solvents and
wastes,
which
came with the semi-synthesis
route. Recent advances in
metabolic engineering
have
created another opportunity to
develop green processes for
relatively complex
molecules.
An example is the production of
shikimic acid for the
synthesis of oseltamivir
phosphate
(Tamiflu) for treatment and
prevention of in.uenza virus
infections. In the
current
ten-step commercial synthesis (Scheme
16.13), the key intermediate
is shikimic
acid
32,
which was produced by a genetically
engineered E. coli strain deficient in
both
shikimate
kinase isozymes or isolated from
the star anise.
The
metabolic engineering efforts
have led to the development
of an E. coli strain
capable
of producing the molecule
with a titer of 84 g L-1 and a yield of 33%
from
glucose.
This acid was subsequently converted
into a diethyl ketal 33, which
was then
transformed
to the oseltamivir phosphate after
side chain installation,
reductive opening
of
the ketal, base-catalyzed epoxide
ring closure (34)
followed by aziridination (35).
One
drawback
of the above process is the
use of the hazardous azide reagent to
prepare the
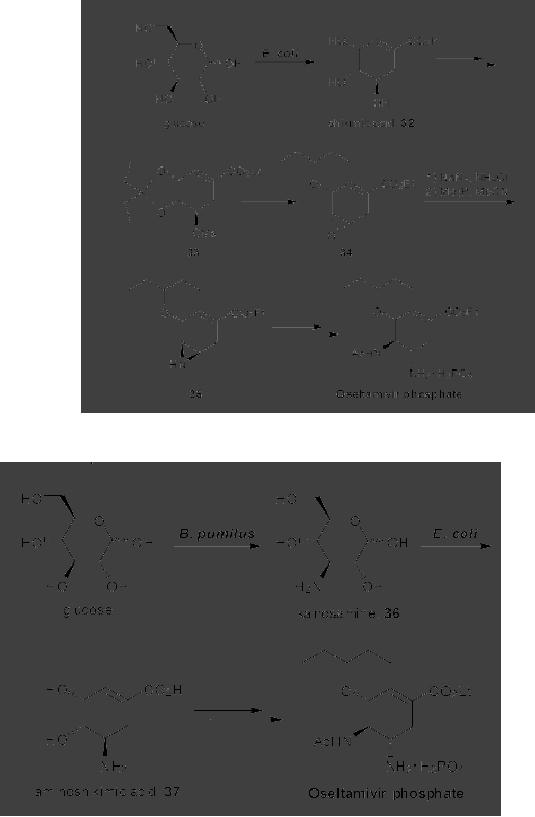
Synthetic
Strategies in Chemistry
16.41
aziridine
intermediate 35. To
eliminate the use of azides, an
azide-free chemoenzymatic
synthesis
of oseltamivir phosphate was recently
reported that comprised
fewer synthetic
steps
than the commercial
process.
Scheme
16.13.
Scheme
16.14.
16.42
Some
Conceptual Developments in Synthesis in
Chemistry
In
this method, the key
intermediate is aminoshikimic acid (37) that
was produced from
glucose
by a two-step microbial process
using Bacillus pumilus to
generate kanosamine
36
followed
by an engineered E. coli to give 37
(Scheme
16.14). The new route
has an
overall
yield of 22% from
aminoshikimic acid and holds great
potential if the
biosynthesis
of aminoshikimic acid can be further
improved.
CASE
STUDY 6: Fine Chemicals
Due
to the exquisite regioselectivity
under mild conditions,
biotransformations often
offer
great advantages over chemical
synthesis in large-scale production of
fine
chemicals,
which is usually energy
intensive and generates a large
amount of wastes and
gas
emissions. The first example of
enzymatic manufacture of a bulk
chemical involves
the
conversion of acrylonitrile to
acrylamide, which is the
monomer of widely
used
polyacrylamide.
The chemical manufacturing
process involves hydration of
acrylonitrile
at
70120oC
by Raney copper resulting in a large
volume of toxic wastes and
HCN. In
addition,
the acrylamide produced by
this fashion requires considerable
purification as it
tends
to polymerize under the
harsh reaction conditions.
Acrylic acid is also a byproduct
of
chemical hydration. Many
nitrile hydratases catalyze the
conversion of acrylonitrile
into
acrylamide.For example, immobilized
Rhodococcus rhodochrous J1 was able
to
produce
acrylamide at a concentration of 400 g
L-1 in a
fed batch process under 10
.C.
There
is no need to recover residual
acrylonitrile because the
yield of the
enzymatic
conversion
is almost 100%. Currently
the microbial process is
operated at a scale of
>40
000
t year-1. It is much greener and more
economical than the chemical
process. In
addition
to acrylamide, Rhodococcus rhodochrous J1
was also used to prepare a
variety
of
amides in high concentration and
throughput in water (Fig.
16.1). The drive
toward
environmentally
benign synthesis and sustainable
development in the chemical
industry
is
contributing to a growing interest in
the use of renewable feed stocks and
reduction of
hazardous
wastes in manufacturing. Consequently,
carbohydrates such as glucose
derived
from
corn starch or cellulosic
biomass provide an intriguing
alternative to the current
use
of
nonrenewable petroleum as starting
materials. The keys to the
success of this
transition
are
the elaboration of new
synthetic routes, as well as
the design and engineering of
robust
microbial biocatalysts. Two examples
are the development of
biocatalytic
syntheses
of adipic acid and catechol from
glucose. Adipic acid , one of the
monomers
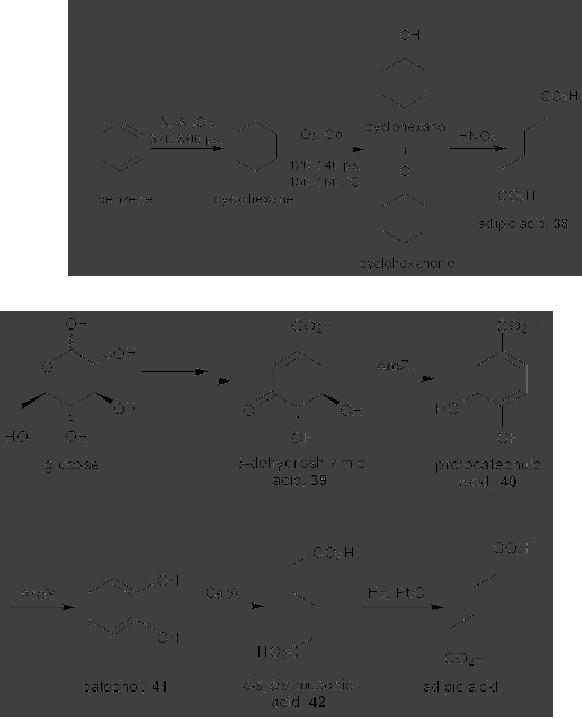
Synthetic
Strategies in Chemistry
16.43
used
in the manufacture of nylon
6,6, is currently produced at
2.2 million metric tons
per
year.
The existing route to adipic
acid first entails oxidation of
cyclohexane, mostly
derived
from benzene, to a mixture of
cyclohexanol and cyclohexanone by oxygen
with a
cobalt
catalyst at a temperature of 150160
�C. This mixture was then
converted to adipic
acid
by oxidation in nitric acid (Scheme
16.15).
Scheme
16.15
Scheme
16.16
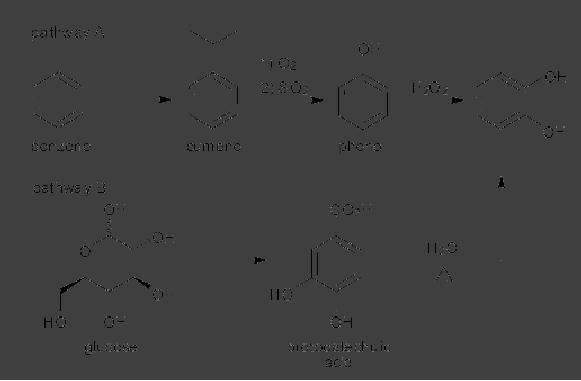
16.44
Some
Conceptual Developments in Synthesis in
Chemistry
Due
to its massive scale, adipic acid
manufacture has been estimated to
account for some
10%
of the annual increase in
atmospheric nitrous oxide
levels.
Benzene
is a carcinogen and volatile chemical and
is derived from
nonrenewable
fossil
fuels. Biocatalytic routes to
adipic acid have been
reported in which glucose
was
converted
to cic,cis-muconic acid followed by
hydrogenation (Scheme 16.16).
The
biosynthetic
pathway leading to the
production of cic,cis-muconic acid was
assembled by
expressing
Klebsiella pneumoniae aro Z-encoded
3-dehydroshikimate dehydratase,
aroY-
encoded
protocatechuate decarboxylase,44c
and
Acinetobacter calcoaceticus catA-
encoded
catechol 1,2-dioxygenase in a
3-dehydroshikimate-syntheszing E. coli
strain.
The
resulting heterologous biocatalyst E.
coli WN1/pWN2.248 was able to produce
37
gL-1 of
cic,cis-muconic acid (42) in 22%
yield from glucose in
one-pot via intermediates
39, 40
and
41
(Scheme
16.16). Catalytic hydrogenation of
cic,cis-muconic acid affords
adipic
acid in 97% yield.
Scheme
16.17.
In
the above metabolic pathway,
catechol is an intermediate in the
route to cic,cis-
muconic
acid. As a result, this technology
may also be applied to the
production of
catechol,
which is manufactured at an annual
volume of 25000 metric tons as
an
important
chemical building block for
flavors, pharmaceuticals,
agrochemicals,
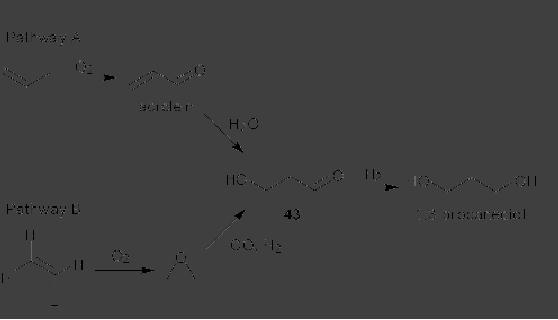
Synthetic
Strategies in Chemistry
16.45
polymerization
inhibitors and antioxidants. Although
some catechol is distilled
from coal
tar,
phenol is the starting
material for the production
of the majority of catechol, and
was
obtained
by Hock-type air oxidation of
benzene-derived cumene (pathway A,
Scheme
17).47 Direct
microbial synthesis of catechol
from glucose resulted in
only 5% yield due
to
its high toxicity to the
production host. The issue
was circumvented by microbial
production
of a less toxic intermediate
protocatechuate using E. coli
KL3/pWL2.46B
followed
by chemical decarboxylation in water
(pathway B, Scheme
16.17).
By
this strategy, the overall
yield of catechol from
glucose was improved to 43%.
As
illustrated
in microbial syntheses of adipic acid and
catechol, manufacture of
chemicals
of
major industrial importance
from renewable feedstocks offer
significant environmental
advantages
and economic opportunities. A single
genetically engineered microbe is
able
to
catalyze the conversion of
glucose in water at near-ambient
pressure and temperature.
The
spectrum of chemicals that
could be synthesized from
glucose can be further
expanded
by the construction of heterologous
biocatalysts and integration of
microbial
and
chemical transformations.
Scheme
16.18.
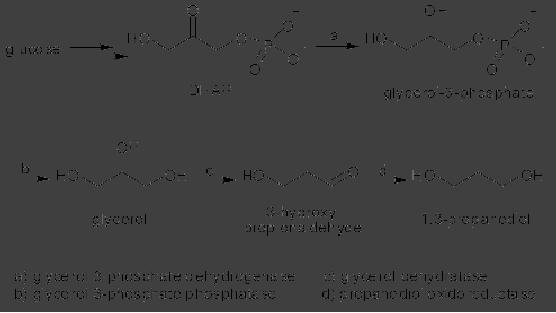
16.46
Some
Conceptual Developments in Synthesis in
Chemistry
Research
aimed at increasing synthetic efficiency
and overcoming product toxicity
and
isolation
problems are critical to
move biocatalytic syntheses from
proof-of-concept into
practical
routes to compete with existing
chemical routes based on
petroleum. Another
example
using metabolic engineering is
microbial production of 1,3-propanediol.
The
emergence
of a new 1,3-propanediol (PDO)-based
polyester has dramatically
increased
the
demand for PDO in recent years.
Once a fine chemical, PDO is
now becoming a bulk
chemical
as its production volume will
increase to a level of one million
tons annually.
Historically,
PDO was produced from
petroleum chemicals from two
different chemical
processes.
One uses propylene as the
starting material, which was
catalytically oxidized
to
acrolein, followed by hydration to
3-hydroxypropionaldehyde (pathway A,
Scheme
18).
An alternative process starts from
ethylene oxide obtained from
oxidation of
ethylene
(pathway B, Scheme 18).
Ethylene oxide was then
converted to 3-
hydroxypropionaldehyde
by hydroformylation under high pressure.
The conversion of 43
to
PDO requires hydrogenation
over a rubidium or nickel
catalyst under
high-pressure.
To
lower the cost of PDO and reduce
the negative environmental
impact of its
production,
a recombinant E. coli strain was
developed to produce PDO
from glucose.
The
E. coli strain was modified to
contain genes from Saccharomyces
cerevisiae, which
is
capable of producing glycerol
from glucose, and Klebsiella
pneumoniae, which
contains
the metabolic pathway of
glycerol to PDO (Scheme
19).
Scheme
16.19.
Synthetic
Strategies in Chemistry
16.47
The
engineered strain relies on a
predominantly heterologous carbon
pathway that diverts
carbon
from dihydroxyactone phosphate (DHAP) to
PDO. Two genes from
yeast
encoding
glycerol 3-phosphate dehydrogenase (dar1)
and glycerol 3-phosphate
phosphatase
(gpp2) directs glycolysis
pathway to glycerol. Two genes
from K.
pneumoniae
encoding
glycerol
dehydratase
(dhaB1-3)
and
1,3-propanediol
oxidoreductase
(dhaT) then transform
glycerol to PDO. Significant
modifications were
then
made to the base strain by
extensive metabolic engineering to
improve the
fermentation
productivity. Additional genes
from dha operon were
incorporated into the
engineered
strain to stabilize glycerol dehydratase
via reactivation. Moreover, an E.
coli
endogenous
oxidoreductase (yqhD) was found to be
superior to dhaT in providing
high
PDO
titer (��130 g L-1). As a
result, the yield of PDO
production in one-pot
from
glucose
was eventually improved to 51% (g
g-1).
In
contrast to the chemical
processes to PDO, the
biocatalytic process uses
a
renewable
resource and was run at close to room
temperature without added
pressure.
The
bioprocess to PDO also reduces
the energy consumption by
40% and green gas
emissions
by 20%. As of December 2006, shipments of
corn sugar-derived PDO
have
been
initiated from a 100 million-lb
per- year plant. The
successful development of a
bio-
based
PDO process showed that it
is possible to produce bulk chemicals in
a more cost-
effective
way than the chemical
processes while adhering to
the principles of green
chemistry.
CASE
STUDY 7: Polymers
Another
recent application of biocatalysis
for green chemistry development is
the
production
of polymeric materials. Enzymatic
polymerization, i.e., in vitro
synthesis of
polymers
using isolated enzymes,
offers a number of advantages
over conventional
chemical
methods that often require
harsh conditions and the use
of toxic reagents.
Benefiting
from high regio and
enantioselectivities under mild
conditions, an enzymatic
approach
provides a new synthetic
strategy for the production
of novel polymers,
which
are
otherwise dif.cult to get by
chemical transformations. Enzymatic
polymerization has
been
applied to the synthesis of a
number of polymers such as
polyesters, polycarbonates,
polysaccharides,
polyurethanes, polyaromatics and vinyl
polymers using
oxidoreductases,
transferases
and hydrolases.
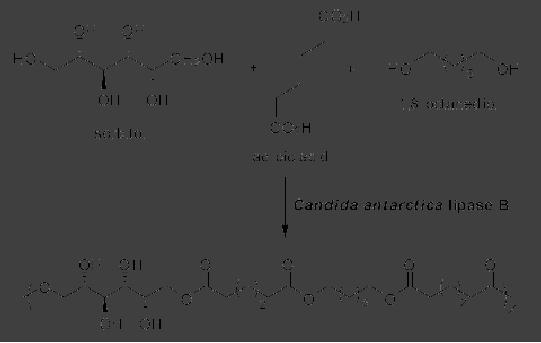
16.48
Some
Conceptual Developments in Synthesis in
Chemistry
For
example, a simple,
environmentally-friendly, and versatile
method was recently
developed
to produce polyol-containing polyesters
through selective
lipase-catalyzed
condensation
polymerization between diacids and reduced sugar
polyols such as
sorbitol
(Scheme
20). Instead of using
organic solvents, the
monomers adipic acid,
glycerol and
sorbitol
were solubilized within
binary or ternary mixtures, and no
preactivation of adipic
acid
was needed. The direct
condensation of adipic acid and sorbitol
was performed in
bulk
at 90 oC
for 48 h using immobilized lipase B
from Candida Antartica (Scheme
20).
The
product, poly(sorbityl adipate), had an
average molecular weight of 10880
(Mn)
and
17030
(Mw)
respectively.
Scheme
16.20
By
replacing a fraction of sorbitol
with 1,8-octanediol, copolyesters of
adipic acid, 1,8-
octanediol,
and sorbitol were obtained in
the molar ratio of 50 : 35 : 15
with an Mw of
>100
000. Similar results were
also obtained with glycerol in place of
sorbitol as the
natural
polyol (not shown). The
condensation reactions with
gycerol and sorbitol
building
blocks
proceeded with high
regioselectivity without
protectiondeprotection of
functional
groups.
Although the polyol monomers
contain three or more
hydroxy groups, only
two
are
highly reactive in enzymatic
polymerization. Therefore instead of
obtaining highly
cross-linked
products, the high
regioselectivity leads to lightly
branched polymers
where
Synthetic
Strategies in Chemistry
16.49
the
degree of branching varies
with the reaction time and
monomer stoichiometry.
The
mild
reaction conditions also facilitate
the polymerization of chemically and
thermally
sensitive
molecules. Alternative chemical
polymerization would necessitate
the use of
stoichiometric
amount of coupling agents.
The biocatalytic approach is also
versatile for
simultaneous
polymerization of lactones, hydroxyacids,
cyclic carbonates, cyclic
anhydrides,
amino alcohols, and hydroxythiols as a
result of high
regioselectivity.
Another
example is enzyme-catalyzed ring-opening
polymerizations of lactones and
cyclic
carbonates, which offer number of
advantages over chemical
methods including
mild
reaction conditions and improved
propagation kinetics and/or
molecular weights.
The
ring-opening polymerization of pentadecalactone and
trimethylene carbonate
catalyzed
by the lipase PS-30 and Novozyme-435 in
bulk at 70 oC
afforded high average
molecular
weight and moderate dispersity products
(not shown).
Enzymatic
polymerization
also allows structural control by
regioselective incorporation of
multifunctional
initiators such as carbohydrates. In
the ring-opening polymerization of
e-
caprolactone
or trimethylene carbonate with ethyl
glucopyranoside as an initiator
catalyzed
by porcine pancreatic lipase, the
polymerization occurred selectively
from the
6-hydroxyl
position (Scheme 21). Since both
ethyl glucopyranoside and the
monomers
are
liquids, the reaction could
be carried out in the
absence of solvents. The
novel
amphiphilic
product was prepared in one-pot
without the need of
protection and
deprotection
chemistry. Hydrolases were also able to
catalyze transesterification
reactions
between
a monomer and a polymer or between
two homopolyesters that
differ in main
chain
structure. For example,
Novozym-435 catalyzed transesterification
between
polypentadecalactone
(4300 g mol-1) and polycaprolactone
(9200 g mol-1) resulted
in
random
copolymers
within
one
hour.
In
addition
to
catalyzing
metal-free
transesterification
under mild conditions,
lipases endow transesterfication
reactions with
remarkable
selectivity, allowing the
preparation of block copolymers
that have selected
block
lengths. Recent advances in
biocatalysis have also made an in road in
the
manufacture
of biodegradable plastics such as
polyhydroxyalkanoates (PHA) and
polylactides
(PLA). Polyhydroxyalkanoates are a
class of natural polyesters
produced by
many
bacteria in the form of intracellular
granulates as a carbon and energy
reserve.
Since
the initial discovery of
poly-3-hydroxybutyrate (PHB), more
than 130 hydroxyacid
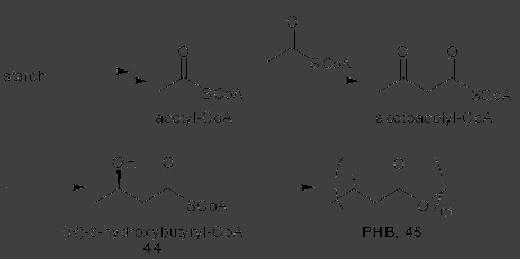
16.50
Some
Conceptual Developments in Synthesis in
Chemistry
monomer
compositions have been
identified that have been
classified as short-chain and
medium-chain
hydroxyalkanoates. Once extracted
from bacterial cells, the
biobased
polymers
display diverse material properties
that range from thermoplastics to
elastomers
depending
upon their monomer unit
composition. The environmental
benefit of using
biodegradable
PHA and the utilization of
abundant, renewable starch as a
starting
material
for its synthesis provide an
appealing alternative to the
commonly used
petroleum-derived
plastics. Of all the PHAs,
PHB copolymers are the
most extensively
studied.
The biosynthetic pathway of
polyhydroxybutyrate consists of three
enzymatic
reactions
(Scheme 22).Thiolase catalyzes the
condensation of two molecules of
acetyl-
CoA
to form one molecule of acetoacetyl-CoA,
which is then reduced to
(R)-3-
hydroxybutyryl-CoA
by
aNADPH-dependent acetoacetyl-CoA dehydrogenase.
The
crucial
polymerization of 44
is
catalyzed by a PHB synthase
with concomitant
regeneration
of coenzyme A (Scheme 16.22).
Scheme
16.22.
PHB
homopolymer is a stiff and brittle
material. The high melting
temperature of PHB
limits
the ability to process the
biopolymer. Incorporation of
3-hydroxyvalerate into
PHB
renders
a poly(3-hydroxybutyrate-co-3-hydroxyvalerate) block
copolymer 48, which
can
be
processed at a lower temperature
while still retaining
excellent mechanical
properties.
The
monomer (R)-3-hydroxyvaleryl-CoA 47
is
produced from condensation of
acetyl-
CoA
and propionyl-CoA to 3-ketovaleryl-CoA (46)
followed by reduction catalyzed
by
acetoacetyl-CoAdehydrogenase
(Scheme 23). With the
advances in genome sequencing
projects,
novel PHA synthases with
different activities and substrate
specificities have
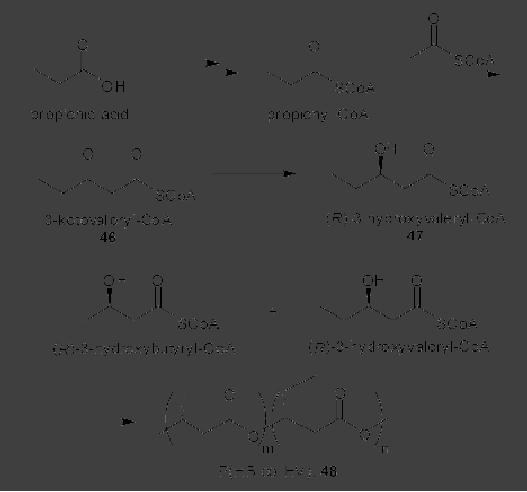
Synthetic
Strategies in Chemistry
16.51
been
identified. Together with
metabolic engineering of diverse,
heterologous pathways
for
efficient biosynthesis of the
monomer substrates from
renewable starting
materials,
high
yielding production of
polyhydroxyalkanoates has been
achieved via
microbial
fermentation.
Accumulation of PHA in genetically
engineered Pseudomonas reached
90%
of the cell weight. Typical
molecular weight of the
polymer ranges from 50 000 to
1
000
000 Da. Recent research
efforts have been devoted to
the production of PHA
in
transgenic
plants. Direct production of
PHA in plants has the
potential to dramatically
lower
the manufacturing cost, and reduce
the burden of plastic wastes
as a result of
biodegradability
to environmentally friendly
products.
Scheme
16.23.
CONCLUSIONS
The
new development in synthetic
chemistry is green chemistry, and the
principles
involved
have created newer
opportunities to develop new
technologies to improve
chemical
processes. A recent study
shows that among the 1039
chemical transformations
16.52
Some
Conceptual Developments in Synthesis in
Chemistry
analyzed
for the synthesis of 128
drug molecules, chemical
acylations are one of the
most
common
transformations which, however,
are generally inefficient.
Development of
catalytic,
low waste acylation methods
would significantly improve
the environmental
performance
of many syntheses. The discovery of
new regioselective and
catalytic
oxidations
would greatly increase
flexibility in synthetic design, as
illustrated in the green
and
biocatalytic synthesis of pharmaceuticals
and fine chemicals. Biotransformations
are
uniquely
suited to deliver high stereo- and
regioselectivity in water at
ambient
temperature
and atmosphere, and are able to catalyze
reactions that are
challenging for
traditional
chemistry. The key is to
integrate synthetic chemistry,
biological
transformations
and process development and biocatalysis
is positioned to be a
transformational
technology for chemical
production with improved
synthetic efficiency.
REFERENCES
1.
James A. Schwarz, Cristian
Contescu & Adriana Contescu,
Chem.Rev., (1995)
95477
2.
Ningqing Ran, Lishan Zhao,
Zhenming Chenb & Junhua
Tao, Green Chem.,
10
(2008) 361.
3.
C. N. R. Rao, Materials Science and
Engineering, BI8 (1993)
1.
4.
Abraham Sz�ke, William G.
Scott & J�nos Hajdu, FEBS
Letters 553 (2003)
18.
5.
The Way of Synthesis:
Evolution of Design and Methods
for Natural Products
Tomas
Hudlicky, Josephine W. Reed, 2007,
Wiley-VCH.
6.
Synthesis of Inorganic Materials,
Ulrich Schubert, Nicola
H�sing, 2005,Wiley-VCH.
7.
H�rk�nen et al., Nucl.
Instr. and Meth. A 541 (2005)
202.
8.
leksic et al., Ann. of NY
Academy of Sci. 972 (2002)
158.
9.
Lindstr�m et al., Nucl.
Instr. and Meth. A 466 (2001) 308 and
cited literature
therein.
10.
C. N. R. Rao Materials Science and
Engineering B,Volume 18,
Issue 1, 1993, 1.
11.
Insights into Speciality
Inorganic Chemicals, David
Thompson, 1995,
Wiley-VCH.
12.
Organic Chemistry Principles and
Industrial Practice, Mark M Green,
Harold A
Wittcoff,
2003, Wiley-VCH.
Table of Contents:
- INTRODUCTION TO SYNTHETIC STRATEGIES IN CHEMISTRY:POROUS MATERIALS
- SYNTHETIC METHODS BASED ON ACTIVATING THE REACTANT:HALOGENATION OF BENZENE
- METHODS BASED ON ACTIVATING THE REACTING SUBSTANCE:Experimental method
- SYNTHESIS OF MATERIALS BASED ON SOLUBILITY PRINCIPLE
- SOL-GEL TECHNIQUES:DEFINITIONS, GENERAL MECHANISM, INORGANIC ROUTE
- TEMPLATE BASED SYNTHESISSynthesis, Mechanism and Pathway
- MICROEMULSION TECHNIQUES:Significance of Packing Parameter
- SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS
- NEWER SYNTHETIC STRATERGIES FOR NANOMATERIALS:Nanostructured Materials
- THE ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY:The Holy Bible
- ELECTROCHEMICAL SYNTHESIS:FEATURES OF ELECTROCHEMICAL SYNTHESIS
- NEWER REACTIONS AND PROCEDURES: CATALYTIC AND NONCATALYTIC
- SYNTHETIC STRATEGIES - FROM LABORATORY TO INDUSTRY
- SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice
- CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES
- SOME CONCEPTUAL DEVELOPMENTS IN SYNTHESIS IN CHEMISTRY
- COMPUTATIONAL BASICS UNDERLYING SYNTHETIC STRATEGIES