 |

Chapter
- 3
METHODS
BASED ON ACTIVATING THE REACTING
SUBSTANCE
R.
Mahalakshmy
1.
INTRODUCTION
The
art of carrying out
efficient chemical transformations is a
major concern in modern
organic
synthesis. Two aspects are of utmost
importance when considering
the outcome of a
reaction,
viz., selectivity and
efficiency (optimization of
yields).
The
new procedures
developed
should also be compatible with
our environment and thus
facilitate preserving
our
resources.
The right activation mode
is, therefore, development of methods
that promote an
efficient
chemical transformation. In this
article, activation should be
understood in a rather
wide
sense. In one-way it is described as a
sort of catalysis facilitating
the course of the
reaction
by lowering the activation
energy. Another way it is
explained in relation to
non-
catalytic
path.
Activation
mode
Physical
Chemical
(i)
Microwave
(ii)
Ultrasound
Catalytic
Non-catalytic
Aqueous
(iii)
High Pressure
Ionic
liquid
(i)
Solvophobic
(i)
Homogeneous
(ii)
Heterogeneous
Supercritical
(ii)
Solvent free
(iii)
Biocatalysis
Micelles
(iii)
Physicochemical
(iv)
Photocatalysis
Micro
emulsion
Electrochemical
Scheme
3.1. Types of activation
mode
Activation
is achieved in different ways,
which may be classified into
physical, chemical or
biochemical,
physicochemical modes. Although
exhaustivity is not the aim,
this article tries
to
give a survey on the most recent
advances in either traditional
modes (pressure,
light,
3.2
Methods
Based on Activating the Reacting
Substance
chemical
catalysis) or in novel techniques
(microwaves, sonication,
biocatalysis).
The
various
activation modes discussed in
this article are shown in
scheme 3.1.
2.
PHYSICAL METHODS FOR THE ACTIVATION OF
REACTING
SUBSTANCE
2.1.
Activation by Microwave
Microwaves
are a form of electromagnetic waves
(wavelength between 1cm and
1m). When
molecules
with a permanent dipole are
placed in an electric field,
they become aligned
with
that
field. If the electric field
oscillates, then the
orientations of the molecules will
also
change
in response to each
oscillation.
Most
microwave ovens operate at 2.45
GHz,
wavelength
at which oscillations occur
4.9 x109 times
per second. Molecules
subjected to
this
microwave radiation are
extremely agitated as they
align and realign themselves
with the
oscillating
field, creating an intense
internal heat that can
escalate as quickly as 10°
C
per
second.
This technique proves to be
excellent in cases where
traditional heating has a
low
efficiency
because of poor heat transmission
and, hence, local overheating is a
major
inconvenience.
Microwave
energy is fast becoming the
method of choice for both
industrial and
academic
chemists for driving
reactions to completion, as it offers
the safest, most
effective
way
to increase reaction rates and
improve product yields,
while promoting green
chemistry.
Reactions
that previously took hours,
or even days, to complete can
now be performed in
minutes.
Decreasing reaction times
offers teaching opportunities: students
have more time
for
design, optimization, characterization
and analysis of reaction
processes and
products.
Additionally,
microwave-assisted reactions are
often performed in aqueous solutions or
neat,
minimizing
the need for organic
solvents, simplifying the
work-up process, and
providing
"green"
reaction conditions.
Is
microwave assisted organic
synthesis green and
safe?
It's
time to think of the
environment and our impact
on it. Microwave energy is an
inherently
efficient
way to transfer energy to a
reaction, as it transfers kinetically
rather than
thermally.
Because
of this quality, it is the
ideal energy source for
driving reactions and it
also has the
following
advantages.
Use
water, ethanol or other
environmentally benign
solvents
·
Neat
reactions/high conversions help
eliminate waste
·
Non-hazardous
reagents help students design safer
syntheses
·
Use
catalysts, not stoichiometric
reagents
·
Synthetic
Strategies in Chemistry
3.3
Not
only is microwave-assisted chemistry is
good for the environment, it
is also safer for
chemists.
Microwave synthesis systems designed
for the laboratory offer
an
Unmatched
level of safety.
·
Eliminate
hot plate burns
·
Reactions
return to room temperature
before removing from
microwave
·
A
few representative examples of
microwave assisted chemical
conversions are
considered.
(i)
Oxidation of Primary and Secondary
Alcohol
A
fast and facile microwave
accelerated oxidation of primary alcohols
to carboxylic acids
and
secondary alcohols to ketones were
carried out under
organic/aqueous biphasic
conditions
using 30% aqueous H2O2 in
the presence of sodium
tungstate and
tetrabutylammonium
hydrogen sulfate (TBAHS) as a
phase-transfer catalyst[1]. The
experimental
procedure involves a simple
mixing of an alcohol, Na2WO4, 2H2O and
TBAHS
followed
by the addition of 30% aqueous
H2O2 in
25:1:1:125 molar ratios for
primary and
25:1:1:40
molar ratio for secondary
alcohols in an open vessel. Then
the reaction mixtures
were
placed inside a monomode
microwave reactor and
irradiated under a reflux
condenser
for
specific time. The best
results were obtained when
the temperatures of reaction
mixtures
were
set to 90° C and 100° C
for primary and secondary
alcohols, respectively.
O
OH
OH
H2O2,
MW, 20 min
Na2WO4,
TBAHS
(Yield=
84%)
O
OH
H2O2,
MW, 10 min
Na2WO4,
TBAHS
(Yield=
92%)
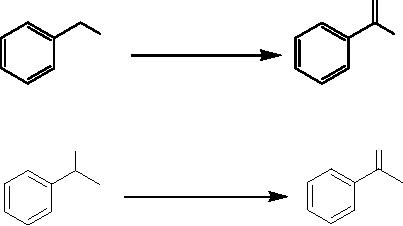
(ii)
Nucleaphilic Substitution
Reaction
The
synthesis of arylamines through direct
nucleophilic substitution of aryl
halides typically
requires
highly polar solvents such
as DMF and DMSO at high temperatures
even with
highly
activated aryl halides. A
novel and efficient synthesis of
N-arylamines by the
reaction
of
activated p-bromonitrobenzene with
secondary amines (morpholine and
N-phenylpi-
3.4
Methods
Based on Activating the Reacting
Substance
perazine)
in the presence of basic Al2O3 under
microwave irradiation was
carried out under
solvent-free
conditions in a simple domestic
oven [2].
O2N
N
O
Al2O3,
3 min
Br
+ HN
O2N
O
MW
(350W)
Yield=
80%)
Both
alumina and amines are polar
so they absorb microwaves
effectively and
consequently
the
reaction is completed in a short
time.
Thus,
the microwave irradiation
has been successfully applied in
organic chemistry.
Spectacular
accelerations, higher yield
under milder reaction
conditions and higher
product
purities
have all been achieved so
far. More over, by using
this technique, a number
of
reactions
has been carried out
successfully that do not
occur by conventional heating
and
even
modifications of selectivity (chemo,
regio and stereoselectivity)
are obtained.
2.2.
Activation by Ultrasound
Ultrasounds
are acoustic waves with frequencies
ranging from 20 to 100 MHz.
They are
mechanical
waves that are not absorbed by
solid and, therefore, they
do not induce
heating.
They
are transmitted through any
substances solid, liquid or
gas, which possesses
elastic
properties.
How
does ultrasound accelerate a chemical
reaction?
The
energy of ultrasound is insufficient to
cause chemical reactions,
but when it travels
through
media a series of compressions and
rarefactions are created, the
rarefaction of liquids
leading
to cavities. During rarefaction,
the negative pressure
developed by the power
of
ultrasound
is enough to overcome the
intermolecular forces binding the
fluid and tear it,
producing
cavitation bubbles. The succeeding
compression cycle can cause
the micro bubbles
to
collapse almost instantaneously
with the release of large
amounts of energy.
The
enormous
rise in local temperatures and
pressures produces a dramatic beneficial
effect of
reaction
acceleration, with relatively
short times being required
for completing the
reaction
such
that the decomposition of
thermally labile products is
minimised.
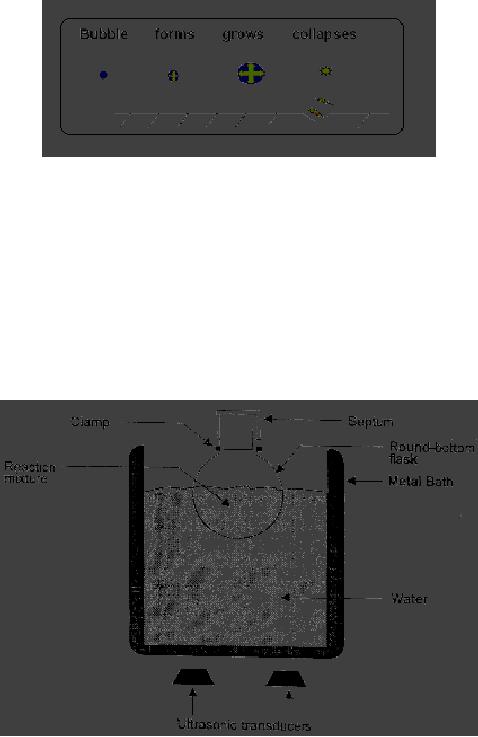
A
schematic
representation
of cavitaional erosion of solid is
given in Fig. 3.1.
Synthetic
Strategies in Chemistry
3.5
Fig.
3.1. Cavitational erosion of
solid
How
to carry out a chemical
reaction in an ultrasonic
bath?
A
number of common reactions
used in synthetic organic
chemistry can be carried out
more
efficiently
using ultrasound. These
reactions can be performed by
immersing a reaction
vessel
into an ultrasonic bath
(Fig. 3.2) or by immersing an
ultrasonic probe (horn) into
a
reaction
medium.
Fig.
3.2. Apparatus for carrying
out a reaction in an ultrasonic
cleaning bath
(Reproduced
from a general article
entitled, "Ultrasound: A Boon in
the Synthesis of
Organic
Compounds"
published in Resonance, 1998,
3,
56)
There
are several advantages of this
method and they are:
(i)
It
increases the yield and
the percentage of by-products
decreases.
(ii)
Reactions
occur faster, so that lower
temperatures can be used.
(iii)
Reaction
times decrease by a factor
five to fifty for identical
isolated yields.
3.6
Methods
Based on Activating the Reacting
Substance
(iv)
It
provides alternative pathways
for reactions, due to the
formation of high-energy
intermediates.
In
the following text several
specific applications of Sonochemistry
are given [3].
(i)
Preparation of organometallic
reagents
Sonochemistry
enables reactions involving
organometallic reagents to be carried
out safely.
The
reaction of magnesium with
ethyl, butyl and phenyl
bromides in aqueous diethyl ether
or
in
n-dibuty1
ether containing 50% benzene
or light petroleum is accelerated by
utilising
ultrasound.
R-Mg-X
R-X
+ Mg
R
= C2H5 ,
nC4H9 , C6H5
X
= Cl, Br
(ii)
Oxidation of alcohol
The
oxidation of alcohols by solid potassium
permanganate in hexane and benzene
is
significantly
enhanced by sonication in an ultrasonic
bath.
R
R
KMnO4
OH
O
(iii)
Reformatsky reaction
The
Reformatsky reaction can be
carried out in high yield
(98 %) in just 30 minutes at
2530o
C
as compared to conventional method, which
gives only 50% yield
after 12 hours at 80o C.
NaX
C4H8C=O
+ BrCH2COOEt + Zn
C4H8C(OH)CH2COOEt
(iv)
Breakdown of polymeric organometallic
compound
The
breakdown of polymeric organotin
fluoxides is carried out by
sonication method.
For
this
reaction, there is 900-fold increase in
rate as compared to conventional
reflux.
[R3SnF]n
nR3SnX
R=Me,
nBu or Ph
X=Cl,
Br, NCO

Synthetic
Strategies in Chemistry
3.7
(*Note:
In the above examples the
symbol
represents
the sonication method)
In
addition to the types of
reactions mentioned, ultrasound
has also been used in the
case of
enzyme
catalysed reactions, polmer
chemistry and coal
liquification.
Extension
of
combination
of sonochemistry with other
specific methods, such as
photochemistry and
electrochemistry
appear to be promising. Since it is an
upcoming and a recent field
of
interest,
there is a great deal more
to explore in ultrasonics as an important
tool in order to tap
its
full potential for the
discovery of new reactions
utilising highly energetic
sound waves.
The
sonochemical boom is turning
out to be a real boon for
synthetic chemistry.
2.3.
Activation by high
pressure
Pressure
represents a mild non-destructive
activation mode, generally
respecting the
molecular
structure by limiting decomposition or
further evolution of the
products. The
specific
effects of high pressure can
be of important value for
organic synthesis. The
kinetic
pressure
effect is primarily determined by
the variation of volume due
to changes in the
nuclear
positions of the reactants
during the formation of the
transition state. Related
to
volume
requirements are steric
effects since the bulkiness of
the molecules involved in
the
transition
state conditions the
magnitude of the steric
interactions.
As
a consequence,
pressure
affects volume changes and
should have an effect on
steric congestion.
As
a mild activation mode,
pressure may be considered of
value in the synthesis of
thermally
fragile molecules, permitting a
lowering of the
temperature.
In
addition, the
selectivity
is generally preserved or even
improved under such
conditions. High-pressure
chemistry
is now recognized as a powerful
method to achieve synthetic
organic reactions,
which
are not readily accessible by
usual means. The
applications of this technique
to
organic
reactions like Diels-Alder
[4] and Cyclo addition
[5] are discussed.
(i)
Diels-Alder reaction
The
high pressure (0.8 GPa)
Diels-Alder reaction of
N-methyl-2(1H)-pyridones with
cyclooctyne
at 90°
C
affords 1:1 cycloadducts in 60 -
80% Yield. No adduct is
recovered at
normal
pressure due to the
extrusion of methyl
isocyanate.
O
H3C
R1
N
R1
+
R2
O
CH3
R2
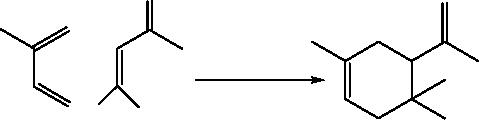
3.8
Methods
Based on Activating the Reacting
Substance
(ii)
Cycloaddition reaction
Cycloaddition
of mesityl oxide to isoprene is
also carried out at high
pressure in
LiClO4/Diethy ether
medium.
O
+
20
MPa, 100 C, 24 h
Dimers
of
+
isoprene
LiClO4/Diethyether
Yield:52%
Yield:19%
When
traditional synthetic strategies appear
forbiddingly difficult or fail
utterly, the pressure
parameter,
possibly associated with
other activation methods may be
considered. Other
salient
features of this method
include:
(i)
Extreme
simplicity of the
method
(ii)
Capacity
to induce ionogenesis
(iii)
Capacity
to remove steric
inhibition
Thus
the notable advantages of this
method materialize in new or
improved synthetic
routes,
and
it adds another important
dimension to the existing
synthetic activation modes.
3.
CHEMICAL METHODS FOR THE ACTIVATION OF
REACTING
SUBSTANCE
3.1.
Non-catalytic activation
3.1.1.
Solvophobic activation
3.1.1a.
Reaction in water
In
the most recent decades, the
use of water as a reaction
solvent or co-solvent has
received
much
attention in synthetic organic
chemistry, with sometimes surprising
and unforeseen
results.
It plays an essential role in life
processes; however its use
as a solvent has been
limited
in organic synthesis. Despite the
fact that it is the
cheapest, safest and most
non-toxic
solvent
in the world, its presence
is generally avoided through
the dehydrative drying
of
substrates
and solvents. But still it
can be considered as a unique
solvent. Moreover, water
is
the
`solvent of Nature' and
therefore the use of water
as a medium for organic
reactions is
one
of the latest challenges for
modern organic chemists.
Synthetic
Strategies in Chemistry
3.9
There
are many potential reasons
to replace the classical organic
solvents by water. They
are
(i)
Cost,
safety and environmental
concern.
(ii)
Aqueous
procedures are often referred to as
green, environmentally friendly,
or
benign.
(iii)
The
unique solvation properties of
water have been shown to
have beneficial
effects
on many types of organic
reactions in terms of both the
rate and selectivity.
(iv)
Experimental
procedures may be simplified, since
isolation of organic
products
and
recycling of water-soluble catalysts and
other reagents can be achieved
by
simple
phase separation.
Selected
few organic reactions run in
an aqueous medium are
considered.
(i) Wittig
Reaction
Bergdahl
and co-workers published the
first report in the
literature describing that
Wittig
reactions
of stabilised (and poorly
water-soluble) ylides with
aldehydes are
unexpectedly
accelerated
in an aqueous medium [6].
COR2
CHO
COR2
Ph3P
R1
R1
a
66-99%
R1= H,
2-NO2, 4-NO2,
2-CN, 4-OH, 4-OMe, 4-NH2, 2-OBn, 3-OBn
R2=
Me, OMe, O-Bu, O-Troc,
Ph
COR2
Ph3P
COR2
R1
1
X
CHO
R
a
X
84-97%
X
= S, NH
R1 = H,
Br, Me, NO2
R2 = OMe,
O-Bu
a)
aldehyde (1 mmol), ylide
(1.2-1.5 mmol), H2O
(5 mL), 20-90ºC, 5 min - 4 h.
Troc= 2,2,2-
trichloroethoxycarbonyl.
(ii)
Mannich-type Reactions
Kobayashi
and co-workers published an
efficient (up to 94% yield)
enantio- and
diastereoselective
protocol for Mannich-type
reactions of a hydrazono ester
with silicon
3.10
Methods
Based on Activating the Reacting
Substance
enolates
in aqueous medium. One
example of a syn
adduct
from an (E)-silicon
enolate and
two
examples of anti
adducts
from (Z)-silicon
enolates are reported
[7].
NHBz
R1
OSiMe3
BzHN
N
a
NH
O
+
EtO
EtO
R2
R3
R3
O
R2
R1
O
R1 = H, Me,
Et, R2=H, Me, R3= Et, S-tBu,
4-Me-C6H4, 4-OMe-C6H4,
4-Cl-C6H4
Ph
Ph
MeO
NH
HN
OMe
b
Where
a = acyl
hydrazono ester (0.4 mmol),
silyl enol ether (1.2
mmol), ZnF2 (100 mol%), b
(10
mol%),
CTAB
(0.02
mmol),
H2O
(1.95
mL),
0
ºC,
20
h.
CTAB=
cetyltrimethylammonium
bromide.
(iii)
Deprotection of Functional
Groups
Methods
for selective deprotection of
functional groups are key
tools for organic
chemists.
The
following examples, performed in
water, open new
possibilities for the use of
this
challenging
medium. Konwar and
co-workers [8] reported a
simple protocol for
the
deprotections
of oximes and imines under
neutral conditions (yields up to
90%) using a
I2/surfactant/water
system
X
a
N
O
R1
R2
R1
R2
X=
OH, Ph
R1,
R2 = alkyl
R1,R2=
aryl
R1= H,
alkyl, R2=aryl
.
Synthetic
Strategies in Chemistry
3.11
where
a= oxime or
imine (1 mmol), I2 (20
mmol%), H2O
(15 mL), SDS (0.2
mmol), 25-
40°C,
3.5-8 h.
The
main obstacle to the use of
water as reaction solvent is
the negligible solubility of
the
majority
of organic compounds in water.
This problem can be
addressed by using aqueous
organic
solvents or phase-transfer agents.
3.1.1b.
Reaction in ionic
liquid
Ionic
liquids are low-melting-point salts
that have attracted
considerable attention recently
as
greener
alternatives to classical environmentally
damaging solvents. "The
interest is mainly
due
to their peculiar properties
such as absence of flammability,
lack of measurable vapor
pressure,
and good ability to dissolve
organic, organometallic, and
even some inorganic
compounds.
These unique chemical and
physical characteristics of ionic liquids
are
increasingly
enticing chemists to explore their
use as media for organic
synthesis.
Ionic
liquids offer numerous advantages
over conventional organic
solvents for carrying
out
organic
reactions. They are,
(i)
Easy
product recovery
(ii)
Catalysts
can be recycled
(iii)
Ionic
liquids can be reused
(iv)
Their
thermodynamic and kinetic
behavior is different
(v)
Rates
of reaction are often enhanced
and
(vi)
Selectivity
is frequently better
Examples
for Ionic
Liquids
The
most common classes of ionic
liquids are alkylammonium
salts, alkylphosphonium
salts
alkylpyridinium
salts, and N, N´-dialkylimidazolium
salts. Few examples of
cationic and
anionic
ionic liquids are given in
Fig. 3.3.
BF4-,
PF6-, SbF6-, NO3-,
R1
R1
CF3SO3-,
(CF3SO3)2N-,
ArSO3-,
CF3CO2-,
CH3CO2-,
Al2Cl7-
N
R2
N
P
R2
R1 NH
N
R2
R3
R3
R3
R3
R
Fig.
3.3. Cationic and anionic
ionic liquids
3.12
Methods
Based on Activating the Reacting
Substance
Synthesis
and applications of halide
based ionic liquid
Halide-based
ionic liquids ILX (IL represents
cations such as 1, 3-dialkylimidazolium,
1-
alkylpyridinium
and tetraalkylammonium; X represents
halide anions) can be used
as
reagents
in nucleophilic substitution for
the conversion of alcohols to
alkyl halides. This
reaction
provides an alternative way of
preparing other types of
ionic liquids (ILA) based
on
the
conjugate bases of acids (HA)
[9].
-H2O
ILA
+ RX
ILX
+ HA + ROH
Where,
ILX:
halide-based ionic liquids;
X=Cl, Br, I; HA: acids; ROH:
alcohols
ILA:
new ionic liquids with
conjugate bases of HA
These
halide-based ionic liquids (ILX)
can also be used as reaction
media for copper-
catalyzed
nucleophilic aromatic substitution
reaction for formation of
aryl nitriles (ArCN).
Cu
catalyst
ArCN
ArY
+ NaCN
ILX,
-NaY
ArY:
Aryl halides, Y= I or Br, ILX:
halide-based ionic
liquid
X=
Cl, Br, I; Cu catalysts:
CuX, X= Cl, Br, I, CN
Trihalide-based
ionic liquids (ILX3)
that can be used as reagents
as well as reaction media
in
halogenation
reactions
-2H2O
ILX
+ 2HX + H2O2
ILX3
ILX:
halide-based ionic liquids, X=
Cl, Br, I
HX:
hydrogen halides
"The
rapid growth of interest in
ionic liquids is mainly
limited to people in academia
and
national
laboratories," "There is a lot of
skepticism among industrial chemists,
probably
because
our understanding of these
materials is limited. Before we
see industrial chemists
enthusiastically
involved in exploring the
field, a lot of work has to
be done. We need
information
on the toxicity and safety
of these materials and their
effect on the
environment,
as
well as an assessment of their
life cycles. Also, we need cost analyses
compared with
existing
technologies. In addition, it is
important to develop a good
database of all the
information
available on ionic liquids.
Unless we have all this
information, the growth will
be
limited
to a few sectors
only."
Synthetic
Strategies in Chemistry
3.13
3.1.1c.
Reaction in supercritical
media
What
is a supercritical fluid?
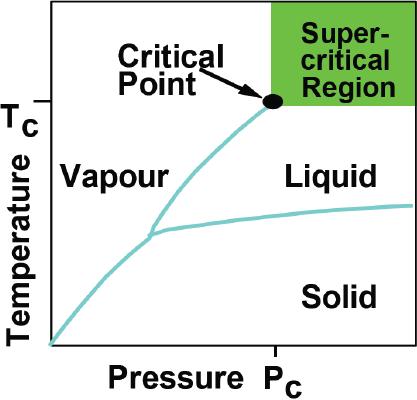
Supercritical
fluids may be defined as the
state of a compound, mixture or
element above its
critical
pressure (Pc) and critical
temperature (Tc), but below
the pressure required
to
condense
it into a solid. It possesses
the characteristics of both fluid
and gaseous
substances:
the
fluid behavior of dissolving
soluble materials, and the
gaseous behavior of
excellent
diffusibility.
They occupy a point where
pure and applied science
meets head on. This is
a
feature
that has attracted many
workers to the field. A
general phase diagram for
critical
fluid
is given in Fig. 3.4.
Fig.
3.4. General phase diagram
for super critical
fluid
(Reproduced
from the web page:
http://www.ed406.upmc.fr/cours/shaldon.pdf)
Reactions
under supercritical conditions
have been used for
large-scale industrial
production
for
most of the twentieth century,
but the application of
supercritical fluids (SCFs) in
the
synthesis
of complex organic molecules is
only just emerging. Research
in this field has
been
particularly
active in the last decade of
this century, because the
following special
properties
of
SCFs make them attractive
solvents for modern
synthetic chemistry.
3.14
Methods
Based on Activating the Reacting
Substance
Increased
reaction rates and
selectivities resulting from
the high solubility of
the
·
reactant
gases
Rapid
diffusion of solvents
·
Weakening
of the solvation around the
reacting species and the
local clustering of
·
reactants
or solvents.
These
fluids are easily recycled
and allow the separation of
dissolved compounds by a
·
gradual
release of pressure
Sequential
and selective precipitations of
the catalyst and product
would be possible.
·
Example
for Carboncarbon bond formation
reactions in supercritical
fluids
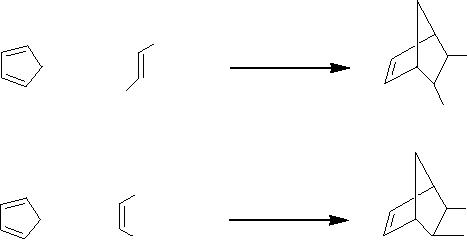
(i)
Diels-Alder
reaction
The
DielsAlder reaction is the most
widely-used synthetic method
for the synthesis of
polycyclic
ring compounds. Kolis et al
[10] have reported the
possibility of performing
DielsAlder
reactions in superheated and
scH2O
due to the unique properties
of scH2O
[11].
The
reactions tested were the
cycloadditions of cyclopentadiene (1)
with diethyl furmarate
(2)
and
diethyl maleate (4) using
scH2O
as the solvent. They
obtained yields of 10 and
86% for 3
and
5, respectively, after 1 h. Although
the yield of the endo/exo-2,
3-diethyl ester of 5-
norbornene
3 was low, equal amounts of
both isomers of 5 were formed in
good yield from
the
cis diene.
CO2Et
scH2O
CO2Et
+
375
oC,
1h
EtO2C
CO2Et
2
3
1
CO2Et
scH2O
CO2Et
+
CO2Et
375
oC, 1h
CO2Et
5
4
1
3.1.2.
Solvent Free or Solid State
Reaction
Synthetic
Strategies in Chemistry
3.15
A
solvent-free or solid-state reaction
may be carried out using
the reactants alone
or
incorporating
them in clays, zeolites,
silica, alumina or other matrices.
Thermal process or
irradiation
with UV, microwave or ultrasound
can be employed to bring
about the reaction.
Solvent-free
reactions obviously reduce pollution,
and bring down handling
costs due to
simplification
of experimental procedure, work up
technique and saving in
labour. These
would
be especially important during
industrial production.
Often,
the products of solid state
reactions turn out to be
different from those obtained
in
solution
phase reactions. This is
because of specific spatial
orientation or packing of
the
reacting
molecules in the crystalline
state. This is true not
only of the crystals of
single
compounds,
but also of co-crystallized
solids of two or even more
reactant molecules. The
host-guest
interaction complexes obtained by
simply mixing the components
intimately also
adopt
ordered structure. The
orientational requirements of the
substrate molecules in the
crystalline
state have provided
excellent opportunities to achieve
high degree of
stereoselectivity
in the products. This has
made it possible to synthesize chiral
molecules
from
prochiral ones either by
complexation with chiral
hosts or formation of
intermediates
with
chiral partners.
Experimental
method
If
two or more substrates are
involved in the reaction,
they are thoroughly ground
together in
a
glass mortar or cocrystallized,
and allowed to stay at room
temperature or transferred to a
suitable
apparatus and heated carefully in an oil
bath or exposed to appropriate
radiation until
the
reaction is complete.
More
sophisticated reaction procedures are
also adopted, if
necessary.
TLC can monitor the
progress of the reaction. In
some cases, a small quantity
of
water
or a catalyst may be added. If it is a
single-compound reaction, it is subjected
to heat or
radiation
directly. Care is to be taken to collect
the volatile products, if
they are produced. In
this
article illustrative examples
representing a number of organic
syntheses performed
under
both
thermal and photochemical
conditions are described
[12].
Examples
(i)
Solid-state reactions are
not really a new concept.
They have been reported even
in
undergraduate
text books. In fact, the
historically significant first
organic synthesis of
urea
by Wöhler achieved in 1828
belongs to this class
Solid
NH4NCO
NH2-CO-NH2
3.16
Methods
Based on Activating the Reacting
Substance
(ii)
Pyrolytic distillation of barium or
calcium salts of carboxylic acids to
prepare ketones is
even
now a commonly used
procedure.
(Ph-CH2-COO)2Ba
Ph-CH2-CO-CH2-Ph +
BaCO3
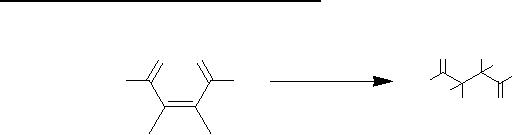
(iii)
Michael Addition
The
addition of a nucleophile to a
carbon-carbon double bond
with a strong
electron-
withdrawing
group at the vinylic
position is known as Michael
addition.
NO2
Al2O3
O
O
+
CH3NO2
Ph
Ph
Ph
Ph
(iv)
Aldol Reaction
Aldol
condensation is an important reaction of
aldehydes and ketones in
forming carbon-
carbon
bonds. The addition of an
enol or enolate ion of an
aldehyde or a ketone to
the
carbonyl
group of an aldehyde or a ketone is
aldol addition, or aldol
condensation, if water is
eliminated
in a subsequent step to produce a, b-unsaturated
aldehyde or ketone.
Many
variations
of this reaction are known
and are called by different
names.
NaOH
Solid
r.t
Ar-CHOH-CH2-CO-Ar' +
Ar-CH=CH-CO-Ar'
ArCHO
+ Ar'-CO-CH3
5
min; 97%
(
In solution only 11% yield
was realized in 5
min)
(v)
Oxidations of alcohol to
ketone/aldehyde
Cr-Oxides
t-Bu
OH
t-Bu
O
Solid
Cr-Oxides
CHO
CH2OH
Solid
Synthetic
Strategies in Chemistry
3.17
3.1.3.
Activation by Physicochemical
Methods
3.1.3a.
Reaction in Micellar
media
Micelles
are dynamic colloidal aggregates
formed by amphiphilic surfactant
molecules.
These
molecules can be ionic,
zwitterionic, or non-ionic, depending on
the nature of their
head
groups, their micelles being
classified in the same way.
In dilute solutions,
amphiphile
molecules
exist as individual species in
the media and these
solutions have completely
ideal
physical
and chemical properties. As
the amphiphile concentration
increases, aggregation of
monomers
into micelles occurs and, as
a consequence, these properties
deviate gradually
from
ideality. This concentration is
called the critical
micellisation concentration.
During
the
formation of micelles, head group
repulsions are balanced by
hydrophobic attractions
and
for
ionic micelles, also by attractions
between head groups and
counterions. Hydrogen
bonds
can
be also formed between adjacent head
groups.
It
is well known that
performing the reactions in
micellar media instead of
pure bulk
solvents
can alter the rates
and pathways of all kinds of
chemical reactions.
Micelles
are
able
to
(a)
Concentrate the reactants
within their small
volumes,
(b)
Stabilise substrates, intermediates or
products and
(c)
Orientate substrates so that
ionization potentials and
oxidationreduction
properties,
dissociation
constants, physical properties, quantum
efficiencies and reactivities
are
changed.
Thus,
they can alter the
reaction rate, mechanism and
the regio- and
stereochemistry. For
many
reactions, rate increments of
5100-fold over the
reactions in homogeneous solutions
have
been reported. In some cases, rate
increments may be higher and
increments in the
order
of
106-fold have been
observed.
Example
(i)
Ring opening reaction of
epoxide
The
ring opening reaction of
styrene oxide with NaCN
was studied in the micellar
solution of
sodium
dodecyl sulfate (SDS) as an
anionic micelle at different
concentrations in the
presence
of catalytic amounts of Ce(OTf
)[13].
Ph
Micelle(SDS)
PhCH(OH)CH2CN +
PhCH(CN)CH2OH
O
Ce(OTf)4,
Cat, rt, NaCN
92%
8%
3.18
Methods
Based on Activating the Reacting
Substance
The
CMC of SDS= 8.1x10-3 M
Thus,
it is established that, in many
cases, performing the
reactions in micellar media
instead
of
organic solvents can alter
rates and the pathways of
the reactions.
3.1.3b.
Reaction in Microemulsion
Media
When
water is mixed with an
organic liquid immiscible
with water and an
amphiphile,
generally
a turbid milky emulsion is
obtained which, after some
time, separates again into
an
aqueous
and an organic phase. On the
water-rich side, the mixtures consist of
stable
dispersions
of oil droplets in water,
which coagulate with rising
temperature. A sponge like
structure
is obtained if the mixtures
contain approximately equal amounts of
water and oil.
On
the oil-rich side, dispersed
water droplets are found,
which coagulate with
decreasing
temperature.
The size of the domains is a
function of the amphiphile
concentration and the
volume
fractions of water and oil.
Since microemulsions contain
both a polar
component
(water)
and a non-polar component
(oil), they are capable of solubilising a
wide spectrum of
substrates.
The mechanism of solubilisation is
similar to that in micellar
solutions. The
micelles
are replaced by the oil
domains, which are capable
of solubilising all kinds
of
hydrophobic
substances. The solubilisation of
polar substances takes place
analogously
through
the aqueous domains of the
microemulsion.
The
solubilisation capacity of
microemulsions
is generally superior to that of
the micellar solutions and
can therefore, affect
the
rate and course of a certain
reaction.
The
use of microemulsions as media
for organic reactions is a
way to overcome the
reagent
incompatibility problems that are
frequently encountered in organic
synthesis. In this
sense,
microemulsions can be regarded as an
alternative to phase transfer
catalysis. The
microemulsion
approach and the phase
transfer approaches can also
be combined, i.e. the
reaction
can be carried out in a
microemulsion in the presence of a
small amount of phase
transfer
agent. A very high reaction
rate may then be obtained.
The reaction rate in
a
microemulsion
is often influenced by the charge at
the interface and this
charge depends on
the
type of surfactant used. For
instance, reactions involving anionic
reactants may be
accelerated
by cationic surfactants. The surfactant
counterion also plays a
major role for
the
reaction
rate. The highest reactivity
is obtained with small
counterions, such as acetate,
that
are
only weakly polarizable.
Large polarizable anions,
such as iodide, bind
strongly to the
interface
and may prevent other
anionic species to reach the
reaction zone.
Example

Synthetic
Strategies in Chemistry
3.19
(i)
Nucleophilic
substitution reactions were
performed in H2O/CO2 (w/c)
microemulsions
formed
with
an
anionic
perfluoropolyether
ammonium
carboxylate
(PFPE COO-NH4+) surfactant. These reactions
between hydrophilic
nucleophiles
and
hydrophobic
substrates
were
accomplished
in
an
environmentally
benign microemulsion without
requiring toxic organic
solvents or
phase
transfer catalysts.
(ii)
The
reaction between benzyl
chloride and potassium bromide to
form benzyl
bromide
is the first organic
reaction performed in w/c
microemulsion [14].
This
reaction
between a nonaqueous compound
soluble in CO2 and a CO2-insoluble
salt
may be expected to take
place at or near the surfactant
interface.
Cl
Br
+
K+Br-
K+Cl-
+
3.1.4c.
Electrochemical Activation
Reactive
intermediates such as carbocations,
carbanions, radicals and
radical ions can be
electrochemically
generated from various electroactive
species. Those intermediates
may
react
chemically (C) or electrochemically
(E) according to EC, ECE
mechanisms. Anodic
oxidations
produce acidic or electrophilic
species, which can react
with nucleophiles or
(and)
eliminate
protons or electrophiles. Cathodic
reductions afford basic or nucleophilic
species,
which
can react with protons or
electrophiles or (and) eliminate
nucleophiles. In this
way,
using
direct electrolytes can
selectively perform functional
group conversion,
substitution
reactions,
addition reactions, cleavage
reactions and coupling
reactions.
Activation
by
transition
metal catalysts is required when
the organic substrate is not
electroactive or leads to
non
desired reactions. The
metal-catalysed electrosynthesis proceeds
by a double activation:
i)
chemical activation of the
organic substrate by the electrogenerated
active form of a
transition
metal catalyst that
generates an organometallic species
more easily reduced
than
the
organic substrate, ii) followed by
activation by electron transfer of
the organometallic
species
formed in the previous
chemical activation step.
This double chemical
and
electrochemical
activation causes new
reactions to proceed, which involve
either, the
classical
organic reactive species,
produced in any electrochemical
steps (carbanions) or
organometallic
complexes (anionic or neutral) as
the basis of new
reactivity.
Example
3.20
Methods
Based on Activating the Reacting
Substance
Allylations
of aldehyde
Using
a recyclable electrochemical process
(up to five cycles with
excellent yield), a
tin-
mediated
protocol for the allylation
of aldehydes (95-100% yield) is
developed [15].
O
OH
Br
a
+
R
R=
Alkyl, aryl
Where
a = graphite electrode (2.0
V), aldehyde (5 mmol), allyl
bromide (8 mmol), SnCl2 (10
mmol),
H2O
(10 mL), r.t., 6-10
h.
3.2.
Catalytic Method of
Activation
The
word catalysis came from
the two Greek words,
the prefix, cata meaning
down, and the
verb
lysein meaning to split or
break. A catalyst breaks down
the normal forces that
inhibit
the
reactions of the molecules; a
widely accepted definition of catalyst
being, `a substance
that
increases the rate of
approach to equilibrium of a chemical
reaction without itself
being
substantially
consumed in the reaction process'.
Catalysis is the phenomenon of a
catalyst in
action,
wherein lowering of the
activation energy is a fundamental
principle that applies to
all
forms
of catalysis homogeneous, heterogeneous or
enzymatic.
Broadly
catalysis can be divided
into five categories:
(i)
Homogeneous
Catalysis: Both
the reactant and catalysts are present in
the same
phase
(ii)
Heterogeneous
Catalysis: Reactant
and catalysts are present in separate
phase,
the
catalyst is solid and the
reactant either liquid or
gas
(iii)
Bio
Catalysis: Also
known as enzyme-catalysis.
(iv)
Photo
Catalysis: Energy
for reactions is from light
source (hν) (e.g. TiO2
photocatalytic
purification and treatment of
H2O)
3.2.1.
Homogeneous catalysis
Homogeneous
catalysis is a chemistry term
which describes catalysis
where the catalyst is
in
the
same phase (ie. solid,
liquid and gas) as the
reactants and products.
The
hydrolysis of esters by acid
catalysis is an example of this -
all reactants and catalyst
are
dissolved
in water:
CH3CO2CH3(aq) + H2O(l) ↔ CH3CO2H(aq)
+ CH3OH(aq) - with
H+ catalyst.
Synthetic
Strategies in Chemistry
3.21
Example
Hydrogenation
of maleic acid to succinic
acid
O
H
O
O
H
H2,
Pd/C
OH
HO
OH
HO
H
EtOH
H
O
Advantage
and drawbacks
Highly
efficient in terms of slectivity (i.e.
regioselectivity. enantiomeric excesses)
and
·
reaction
rates, due to ther
monomolecular nature.
Catalyst
recovery can be very
difficult (due to the
homogeneous nature of
the
·
solution).
Product
contamination by residual catalyst or
metal species is a
problem.
·
3.2.2.
Heterogeneous Catalysis
Heterogeneous
catalysis is
a chemistry term which
describes catalysis where
the catalyst is
in
a different phase (ie.
solid, liquid and gas,
but also oil and
water) to the reactants
and
products.
Heterogeneous catalysts provide a surface
for the chemical reaction to
take place
on.
Example
(i)
Synthesis of Ammonia by Haber
process
3H2(g) + N2(g) ↔ 2NH3(g)
- catalysed
by Fe(s).
In
the Haber process to
manufacture ammonia, finely
divided iron acts as a
heterogeneous
catalyst.
Active sites on the metal
allow partial weak bonding
to the reactant gases, which
are
adsorbed
onto the metal surface. As a
result, the bond within
the molecule of a reactant is
weakened
and the reactant molecules
are held in close proximity to
each other. In this
way
the
particularly strong triple
bond in nitrogen is weakened
and the hydrogen and
nitrogen
molecules
are brought closer together
than would be the case in
the gas phase, so the
rate of
reaction
increases
(ii)
Hydrogenation of ethene on a solid
support
In
order for the reaction to
occur one or more of the
reactants must diffuse to the
catalyst
surface
and adsorb onto it. After
reaction, the products must desorb
from the surface and
diffuse
away from the solid surface.
Frequently, this transport of
reactants and products
from
one
phase to another plays a
dominant role in limiting
the reaction rate.
Understanding these
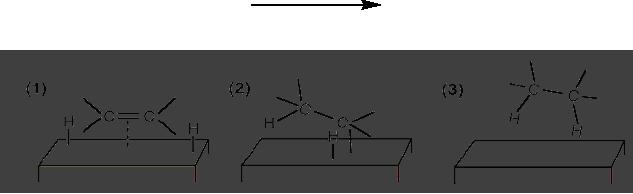
3.22
Methods
Based on Activating the Reacting
Substance
transport
phenomena and surface chemistry
such as dispersion is an important
area of
heterogeneous
catalyst research. Catalyst surface
area may also be considered. A
pictorial
representation
of hydrogenation of ethane on solid
support is shown in Fig.
3.5.
Ni
or Pd catalyst
CH2=CH2
CH3-CH3
Fig.
3.5. Pictorial representation of
hydrogenation of ethene on solid
surface
(Reproduced
from the web page:
http://en.wikipedia.org/wiki/Heterogeneous_catalysis)
Advantages
and drawbacks
Heterogeneously
catalyzed reactions allow
easy and efficient
separation of high
value
·
products
from the catalyst and
metal derivatives.
However,
selectivity and rates are
often limited by the
multiphasic nature of
this
·
system
and/or variations in active
site distribution from the
catalyst preparation.
3.2.3.
Enzyme or biocatalysis
Biocatalysis
can be defined as the
utilization of natural catalysts, called
enzymes, to perform
chemical
transformations on organic compounds.
Both enzymes that have been
more or less
isolated
or enzymes till residing
inside living cells are
employed for this
task.
The
most important conversion in the
context of green chemistry is
with the help of
enzymes.
Enzymes are known as biocatalyst
and the transformations are
referred to as
biocatalytic
conversions. Enzymes are now
easily available and are an
important tool in
organic
synthesis. Biocatalytic conversions have
many advantages in relevance to
green
chemistry.
Some of these are:
Most
of the reactions are
performed in aqueous medium at ambient
temperature
·
and
pressure.
Biocatalytic
conversions normally involve
only one step.
·
Protection
and deprotection of functional
groups is not
necessary
·
Synthetic
Strategies in Chemistry
3.23
Reactions
are fast reactions.
·
Conversions
are stereospecific.
·
Special
advantage of biochemical reaction is
that they are
chemoselective,
·
regioselective
and stereo selective.
A
number of diverse reactions
are possible by biocatalytic
processes, which are
catalysed by
enzymes.
The
major six classes of enzymes
and the type reactions
they catalyse are
discussed.
(i) Oxidoreductases: These
enzymes catalyst oxidation-reduction
reactions. This class
includes
oxidases (direct oxidation with
molecular oxygen) and
dehydrogenases
(which
catalyse the direct removal of
hydrogen from one substrate
and pass it on to
a
second substrate.
(ii)
Transferases:
These
enzymes catalyse the transfer of
various functional groups.
Eg.
Transaminase
(iii) Hydrolases:
This
group of enzymes catalyses
hydrolytic reactions. Eg.
Esterase
(esters)
(iv)
Lyases:
These
are of two types, one
which catalyses addition to
double bond and
the
other
which catalyses removal of
double bond. Both addition
and elimination of
small
molecules
are on sp3 hybridised carbon.
(v)
Isomerases:
These
catalyse various types of isomerisation,
e.g. racemases,
epimerases
etc.
(vi)
Ligases:
These
catalyse the formation or cleavage of
sp3 hybridised carbon.
The
enzymes are specific in
their action. This
specificity of enzymes may be
manifested in
one
of the three ways:
a.
An enzyme may catalyze a
particular type of reaction,
e.g. esterases
hydrolyses
only ester. Such enzymes are
called reaction
specific.
Alternatively,
an enzyme may be specific
for a particular class of
compounds.
These
enzymes are referred to as substrate
specific, e.g., Urease
hydrolyses
only
urea and phosphatases hydrolyse
only phosphate esters.
b.
An enzyme may exhibit
kinetic specificity. For
example, esterase
hydrolyse
all
esters but at different
rates.
c.
An enzyme may be stereospecific.
For example, maltase hydrolyses
alpha-
glycosides
but not beta-glycosides. On
the other hand emulsin
hydrolyses beta
glycosides
but not the alpha
gluycosides.
3.24
Methods
Based on Activating the Reacting
Substance
d.
It should be noted that a
given enzyme could exhibit
more than one
specificities.
The
oxidation accomplished by enzymes or
microorganisms excel in
regiospecificity,
stereospecificity
and enantioselectivity.
An
unbelievably large number of
enzymatic
oxidations
have been accomplished.
Examples:
(i)
Conversion of alcohol into acetic
acid by bacterium acetic in presence of
air (the process is
now
known as quick-vinegar
process)
Bacterium
acetic
CH3CH2OH +
O2
CH3COOH + H2O
(ii)
Conversion of sucrose into
ethyl alcohol by yeast (this
process is used for
the
manufacture
of ethyl alcohol.
Invertase
2C6H12O6
C12H22O11
+ H2O
Yeast
Invertase
C6H12O6
2C2H5OH + 2CO2
Yeast
(iii)
Oxidation of Galactose
Galactose
oxidase (GO) is a fungal
enzyme that catalyzes the
two-electron oxidation of D.
galactose
to the corresponding aldehyde
with the concomitant
reduction of molecular
oxygen
to
hydrogen peroxide.
OH
OH
HO
CH2OH
HO
CHO
GOase
O
H2O2
+
+
HO
O
HO
OH
OH
(iv)
Hydroxylation of aromatic
rings
Benzene
undergoes oxidation with Pseudomonus
putida in presence of oxygen
and gives the
cis-diol.
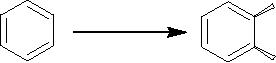
Synthetic
Strategies in Chemistry
3.25
Pseudomonus
OH
Putida
OH
Cis-3,5-cyclohexdiene-1,2-diol
The
path to new chemical
entities often shows the
limitations of existing tools
both in
biocatalysis
and organic chemistry.
Organic synthetic procedures to prepare a
compound in a
target-oriented
synthesis can damage other functional
parts of the molecule.
Protection-
deprotection
schemes can lead to a dead
end, when a certain
protecting group cannot
be
cleaved
off.
In
biocatalysis, on the other
hand, the required
biocatalytic toolbox
and
methodology
might not be readily
available, therefore limiting a
biocatalytic approach.
New
toolboxes,
ingredients, and methodologies at
the interface of classical organic
synthesis and
biocatalytic
reactions bridge the gap
between these two areas.
Since product isolation
and
purification
involves a substantial amount of
time in the preparation of
chemicals,
methodologies
to simplify these tasks are
necessary to get the pure
product into the
bottle
with
less work-up time.
Efficient
and safe new
pharmaceuticals, intermediates and
analytical reagents need to be
prepared
under certain safety,
health, and environmental
and economical
boundary
conditions.
Biocatalytic
reactions have been shown to
overcome these
limitations
successfully
and are becoming
increasingly important in industrial
manufacturing. Building
bridges
between biocatalysis and
organic synthesis will therefore create
roads to new
synthetic
strategies and technological frontiers of
both fundamental and
practical interest.
3.2.4.
Photocatalysis
Photocatalysis
is
the acceleration of a photoreaction in
the presence of a
catalyst.
In
catalysed
photolysis, light is absorbed by an adsorbed
substrate. In photogenerated
catalysis
the
photocatalytic activity (PCA) depends on
the ability of the catalyst
to create electronhole
pairs,
which generate free radicals
(hydroxyl ions; OH-) able to
undergo secondary reactions.
Its
comprehension has been made
possible ever since the
discovery of water electrolysis
by
means
of the titanium dioxide.
Commercial application of the
process is called
Advanced
Oxidation
Process(es) (AOP). There are
several methods of achieving AOP's
that can but do
not
necessarily involve
TiO2 or even the use of UV.
Generally the defining
factor is the
production
and use of the hydroxyl
ion.
Example
(i)
Chlorophyll as photocatalyst
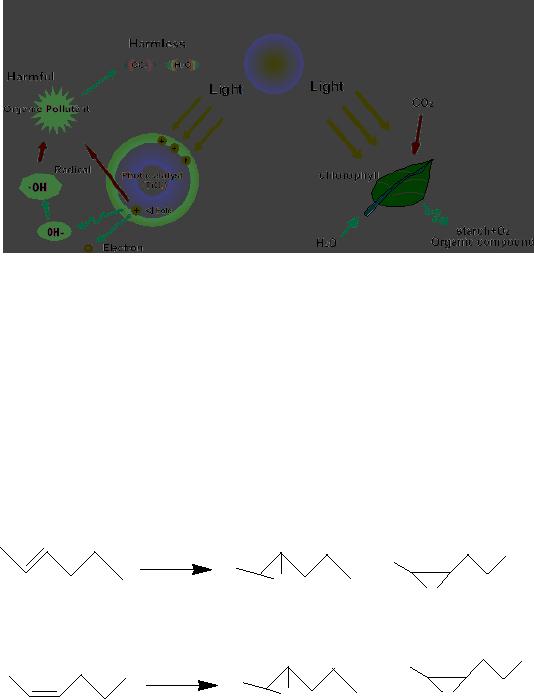
3.26
Methods
Based on Activating the Reacting
Substance
Chlorophyll
of plants is a type of photocatalyst.
Photocatalysis compared to
photosynthesis,
(Figure
6) in which chlorophyll captures sunlight
to turn water and carbon
dioxide into
oxygen
and glucose, photocatalysis
creates strong oxidation agent to
breakdown any organic
matter
to carbon dioxide and water
in the presence of photocatalyst,
light and water.
Fig.
3.6. Comparison of photocatalysis
with photosynthesis
(Reproduced
from the website:
http://www.mchnanosolutions.com/whatis.html)
(ii)
Epoxidation of trans and cis-2-hexene by
TiO2
The
reaction was carried out on
photoirradiated TiO2 powder using
trans-2-hexene or cis -2-
hexene
as the starting material.
From trans-2-hexene, trans-2,
3-epoxyhexane was
obtained
as
the main product with
the ratio between trans and
cis-2, 3-epoxyhexane being
98.4 to 1.6.
In
the case of cis-2-hexene,
the ratio of trans to cis-2,
3-epoxyhexane was 12.0 to
88.0.
trans-2,3-epoxyhexene
trans-2-hexene
cis-
2,3-epoxyhexene
O2
O
TiO2
O
98.4
1.6
cis-
2,3-epoxyhexene
trans-2,3-epoxyhexene
cis-2-hexene
O2
O
O
TiO2
12.0
88.0
A
large number of recent reports on
the photo catalytic reaction
of organic compounds
have
satisfied
the basic requirement of organic
synthesis, e.g isolation and
identification of
product.
Hence photocatalystic reaction is
novel synthetic tool as well
as activation mode.
Synthetic
Strategies in Chemistry
3.27
4.
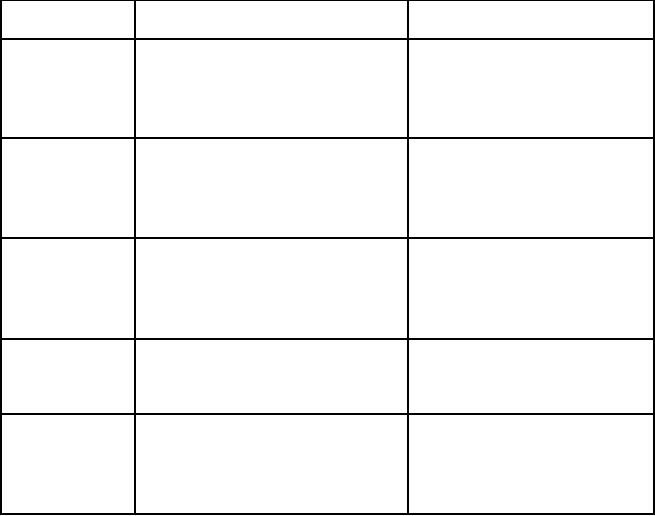
CONCLUSION
The
diversity of activation methods in
organic synthesis has grown
noticeably in recent years.
These
methods have many advantages compared to
traditional techniques. They also
have
some
drawbacks. A summary of advantages and
drawbacks of a few activation
methods,
which
are discussed in this
article are summarized in Table
3.1.
Table
3.1. Summary of features of activation
processes
Activation
mode
Advantages
Drawbacks
Large
volume reactions
Hazardous
temperature control
Fast
reactions (Elimination of volatile
Safety problems (reactions
in
solution)
Microwave
products)
No
reproducibility
Simple
non-destructive method
Low
volume reaction
No
or little work-up
Cost
of equipments
Pressure
Excellent
reproduciblity
Limited
to homogeneous
reactions
(difficult of mixing)
Simple
method
No
generality
Large
volume reaction
Cost
of equipments
Ultrasound
No
or little work-up
Hazardous
temperature control
Adaptable
to heterogeneous reaction
Considerable
acceleration of rate No
generality
Solvophobic
constant,
Possibility
of hydrolysis
interactions
Cheap
environmentally safe
method
Low
temperature efficient
method
No
generality (limited to a
few
Enzymatic
Highly
selective method
specific
reactions)
catalysis
Cost
enzymes
Sensitive
to temperature
This
article shows that the
methods described are fast
and efficient. Relatively
high yields
are
achieved in a very short
time.
The
methods and the results when
compared with
conventional
processes are found to be
inexpensive, more eco-friendly
and high yielding.
Therefore
choosing the correct mode of
activation for a particular
reaction depends
entirely
on
the knowledge of chemists in this
area. In conclusion, this
article reports
important
examples
of activation modes highlighting
the implementation of new
synthetic strategies. It
may
be of great help to chemists of present
and future
generation.
3.28
Methods
Based on Activating the Reacting
Substance
5.
REFERENCES
1.
D. Bogda and M.Ukasiewicz, Synlett,
1 (2000)
143.
2.
J. S. Yadav and B. V. Subba
Reddy, Green
Chemistry,
2
(2000)
115.
3.
V. Singh, K. P. Kaur, A. Khurana
and G. L. Kad, Resonance, 3
(1998)
56.
4.
K. Matsumoto, M. Ciobanu, M. Yoshita
and T. Uschida, Heterocycls, 45
(1997)
15.
5.
G. Jenner and R. Ben Salem, Tetrahedron, 53
(1997)
4637.
6.
J. Dambacher, W. Zhao, A. El-Batta, R.
Anness, C. Jiang and M,
Bergdahl,
Tetrahedron
Lett., 46 (2005)
4473.
7.
T. Hamada, K. Manabe and S.
Kobayashi, J.
Am. Chem. Soc., 126 (2004)
7768.
8.
P.
Gogoi, P. Hazarika, and D.
Konwar, J.
Org. Chem., 70 (2005)
1934.
9.
R.
X . Ren, J.X. Wu, Org.
Lett., 3 (2001)
3727.
10.
M. B . Korzanski, and J. W. Kolis,
Tetrahedron
Lett.,
38
(1997)
5611.
11.
J. Gao, J.
Am. Chem. Soc., 115 (1993)
6893.
12.
G. Nagendrappa, Resonance,
7
(2002)
59.
13.
N. Iranpoor, H. Firouzabadi and M.
Shekarize, Org.
Biomol. Chem.,
1
(2003)
724.
14.
B. Gunilla, C. Jacobson, T. Lee and K. P.
Johnston, J.
Org. Chem., 64 (1999)
1201.
15.
Zha, A. Hui, Y. Zhou, Q.
Miao, Z. Wang and H. Zhang,
Org.
Lett., 7 (2005)
1903.
Books
1.
A.S. Bommarius, B.R. Riebel,
Biocatalysis:
Fundamentals and Application,
Wiley
VCH
Publisher, 2007.
2.
V.K. Ahluwalia, N. Kidwai, New
Trends in Green Chemistry, Kluwer
Academic
Publishers
and Anamaya Publishers,
2004.
3.
M. Kaneko and I. Okura, Photocatalysis:
Science and Technology,
Biological and
medical
physics series, Springer
Publishers, 2003.
Websites
1.
http://www.bentham.org/aos/Samples/aos1-1.htm
2.
http://books.google.co.in
Table of Contents:
- INTRODUCTION TO SYNTHETIC STRATEGIES IN CHEMISTRY:POROUS MATERIALS
- SYNTHETIC METHODS BASED ON ACTIVATING THE REACTANT:HALOGENATION OF BENZENE
- METHODS BASED ON ACTIVATING THE REACTING SUBSTANCE:Experimental method
- SYNTHESIS OF MATERIALS BASED ON SOLUBILITY PRINCIPLE
- SOL-GEL TECHNIQUES:DEFINITIONS, GENERAL MECHANISM, INORGANIC ROUTE
- TEMPLATE BASED SYNTHESISSynthesis, Mechanism and Pathway
- MICROEMULSION TECHNIQUES:Significance of Packing Parameter
- SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS
- NEWER SYNTHETIC STRATERGIES FOR NANOMATERIALS:Nanostructured Materials
- THE ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY:The Holy Bible
- ELECTROCHEMICAL SYNTHESIS:FEATURES OF ELECTROCHEMICAL SYNTHESIS
- NEWER REACTIONS AND PROCEDURES: CATALYTIC AND NONCATALYTIC
- SYNTHETIC STRATEGIES - FROM LABORATORY TO INDUSTRY
- SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice
- CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES
- SOME CONCEPTUAL DEVELOPMENTS IN SYNTHESIS IN CHEMISTRY
- COMPUTATIONAL BASICS UNDERLYING SYNTHETIC STRATEGIES