 |
CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES |
| << SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice |
| SOME CONCEPTUAL DEVELOPMENTS IN SYNTHESIS IN CHEMISTRY >> |
Chapter
-15
CARBOHYDRATES
TO CHEMICALS
Vamsi
Krishna Nunna,
INTRODUCTION
Carbohydrates
are the most abundant
class of organic compounds
found in living
organisms.
They
originate as products of photosynthesis, an
endothermic reductive condensation
of
carbon
dioxide requiring light
energy and the pigment
chlorophyll. Carbohydrates can be
written
as carbon hydrates, Cn(H2O)n,
hence their name.
n
CO2 + n
H2O + energy
CnH2nOn +
n O2
The
carbohydrates are a major source
energy required for
metabolism. Aside from the
sugars
and
starches that meet this
vital nutritional role,
carbohydrates also serve as a
structural
material
(cellulose), a component of the
energy transport compound ATP,
recognition sites
on
cell surfaces, and one of three essential
components of DNA and RNA.
MONOSACCHARIDES
Monosaccharides
can be in turn can be classified as
ketoses and aldoses basing on the
functional
group
present, i.e. ketone or
aldehyde.
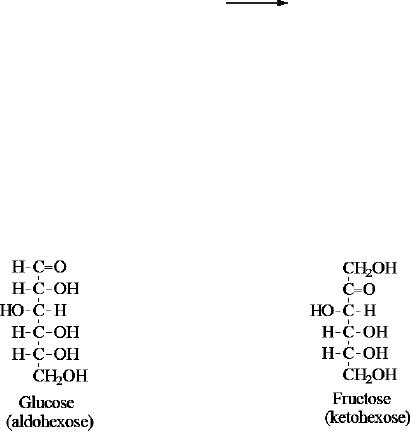
Glucose
is a monosaccharide, an aldohexose and a
reducing sugar. The general
structure of
glucose
and many other aldohexoses was established by
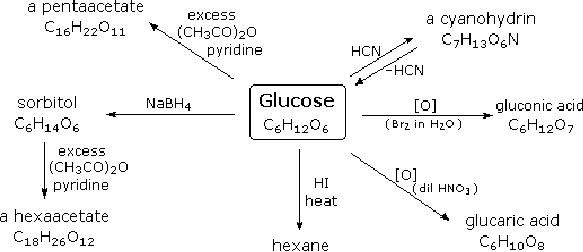
simple chemical
reactions.Hot
hydriodic
acid (HI) was often used to reductively
remove oxygen functional
groups from a
molecule,
and in the case of glucose
this treatment gave hexane
(in low yield). From
this it
was
concluded that the six
carbons are in an unbranched chain.
The presence of an
aldehyde
carbonyl
group was deduced from
cyanohydrin formation, its
reduction to the
hexa-alcohol
sorbitol,
also called glucitol, and mild
oxidation to the mono-carboxylic acid,
glucuronic
15.2
Carbohydrates
to Chemicals
acid.
Somewhat stronger oxidation by
dilute nitric acid gave the
diacid, glucaric acid,
supporting
the proposal of a six-carbon
chain.
Fig.
15.1. Reactions of
glucose
The
five oxygens remaining in
glucose after the aldehyde
was accounted for were
thought to
be
in hydroxyl groups, since a penta-acetate
derivative could be made. These
hydroxyl
groups
were assigned, one each, to
the last five carbon
atoms, because geminal
hydroxyl
groups
are normally unstable
relative to the carbonyl
compound formed by loss of
water.
Glucose
and other saccharides are
extensively cleaved by periodic
acid, thanks to the
abundance
of vicinal diol moieties in
their structure. This
oxidative cleavage, known as
the
Malaprade
reaction is particularly useful
for the analysis of
selective O-substituted
derivatives
of saccharides, since ether functions do
not react. The stoichiometry
of
aldohexose
cleavage is shown in the following
equation.
HOCH2(CHOH)4CHO + 5 HIO4 ----> H2C=O + 5 HCO2H + 5
HIO3
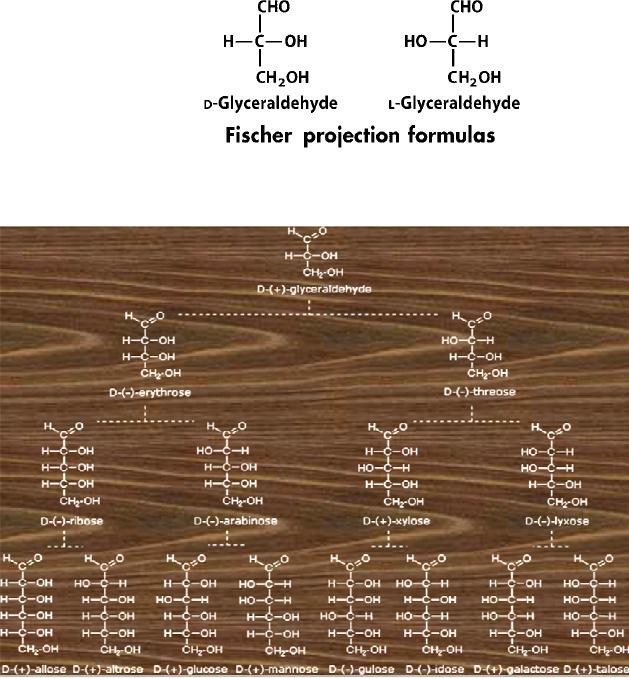
Configuration
of Glucose
The
four chiral centers in glucose
indicate there may be as
many as sixteen (24)
stereoisomers
having
this constitution. These would
exist as eight diasteromeric pairs of
enantiomers, and
the
initial challenge was to
determine which of the eight
corresponded to glucose. This
challenge
was accepted and met in 1891 by the
German chemist Emil
Fischer.
Synthetic
Strategies in Chemistry
15.3
The
last chiral center in an
aldose chain (farthest from
the aldehyde group) was chosen
by
Fischer
as the D / L designator site. If
the hydroxyl group in the
projection formula pointed
to
the
right, it was defined as a member of
the D-family. A left
directed hydroxyl group
(the
mirror
image) then represented the
L-family. It is important to recognize
that the sign of a
compound's
specific rotation (an
experimental number) does
not correlate with
its
configuration
(D or L). It is a simple matter to
measure an optical rotation
with a polarimeter.
Determining
an absolute configuration usually
requires chemical interconversion
with known
compounds
by stereospecific reaction paths.
Fischer
projection formulas and names
for the D-aldose family
(three to six-carbon
atoms)
are
shown below.
15.4
Carbohydrates
to Chemicals
Emil
Fischer made use of several
key reactions in the course of
his carbohydrate studies.
These
are described here
Oxidation
Sugars
may be classified as reducing or
non-reducing based on their
reactivity with
Tollens',
Benedict's
or Fehling's reagents. If a sugar is oxidized by
these reagents it is called
reducing,
since
the oxidant (Ag(+) or Cu(+2))
is reduced in the reaction, as evidenced
by formation of a
silver
mirror or precipitation of cuprous
oxide. The Tollens' test is
commonly used to detect
aldehyde
functions; and because of the
facile interconversion of ketoses and
aldoses under
the
basic conditions of this
test, ketoses such as
fructose also react and are classified
as
reducing
sugars.
1.
2.
3.
Synthetic
Strategies in Chemistry
15.5
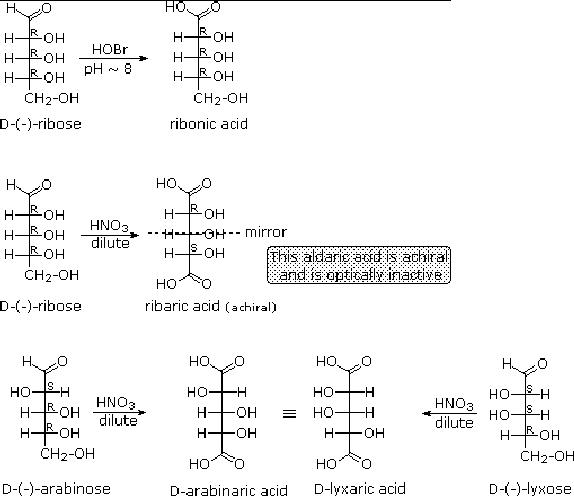
When
the aldehyde function of an
aldose is oxidized to a carboxylic acid
the product is called
an
aldonic acid. Because of the 2�
hydroxyl functions that are
also present in these
compounds,
a mild oxidizing agent such as
hypobromite must be used for
this conversion
(equation
1). If both ends of an
aldose chain are oxidized to
carboxylic acids the product
is
called
an aldaric acid. By converting
aldoses to its corresponding
aldaric acid derivative,
the
ends
of the chain become
identical .
Thus,
ribose, xylose, allose and galactose
yield achiral aldaric acids
which are, of course,
not
optically
active. The ribose oxidation is
shown in equation 2 below.
Other aldose sugars
may
give
identical chiral aldaric acid
products, implying a unique
configurational relationship.
The
examples of arabinose and lyxose
shown in equation 3 above illustrate
this result. A
Fischer
projection formula may be
rotated by 180� in the plane
of projection without
changing
its configuration.
Reduction
Sodium
borohydride reduction of an aldose
makes the ends of the
resulting alditol
chain
identical,
HOCH2(CHOH)nCH2OH,
there by accomplishing the
same configurational change
produced
by oxidation to an aldaric acid. Thus,
allitol and galactitol from
reduction of allose
and
galactose are achiral, and
altrose and talose are reduced to the
same chiral alditol.
Derivatives
of HOCH2(CHOH)nCHO
HOCH2(CHOH)nCO2H
(an Aldonic Acid)
HOBr
Oxidation
---->
HNO3 Oxidation
---->
H2OC(CHOH)nCO2H
(an Aldaric Acid)
NaBH4 Reduction
---->
HOCH2(CHOH)nCH2OH
(an Alditol)
Osazone
Formation
The
Osazone reaction was developed and used by
Emil Fischer to identify
aldose sugars
differing
in configuration only at the
alpha-carbon. The equation
shows the general form
of
the
osazone reaction, which
effects an alpha-carbon oxidation
with formation of a
bis-
phenylhydrazone,
known as an osazone. Application of the
Osazone reaction to D-glucose
and
D-mannose demonstrates that these
compounds differ in configuration
only at C-2.
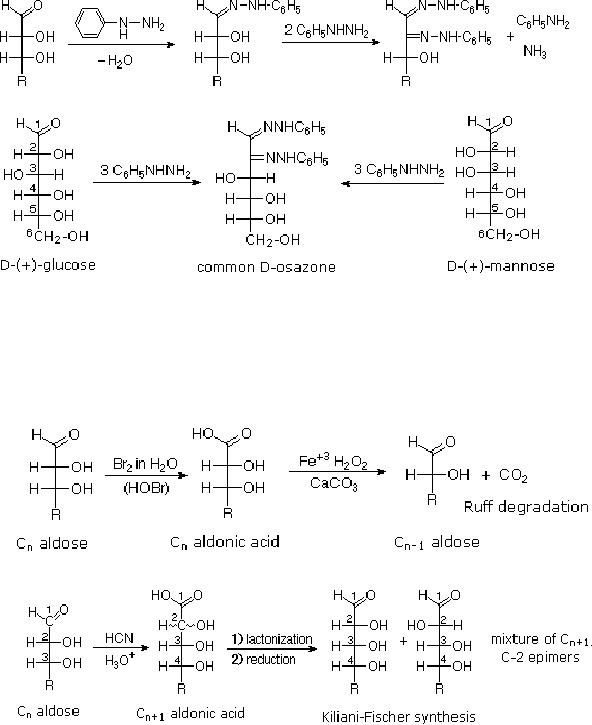
15.6
Carbohydrates
to Chemicals
4.
5.
Chain
Shortening and
Lengthening
6.
7.
These
two procedures permit an aldose of a
given size to be related to homologous
smaller
and
larger aldoses. Thus Ruff
degradation of the pentose arabinose
gives the tetrose
erythrose.
Working in the opposite direction, a
Kiliani-Fischer synthesis applied to
arabinose
gives
a mixture of glucose and mannose. Using
these reactions, we can understand
the
Fischer's
train of logic in assigning
the configuration of
D-glucose.
Synthetic
Strategies in Chemistry
15.7
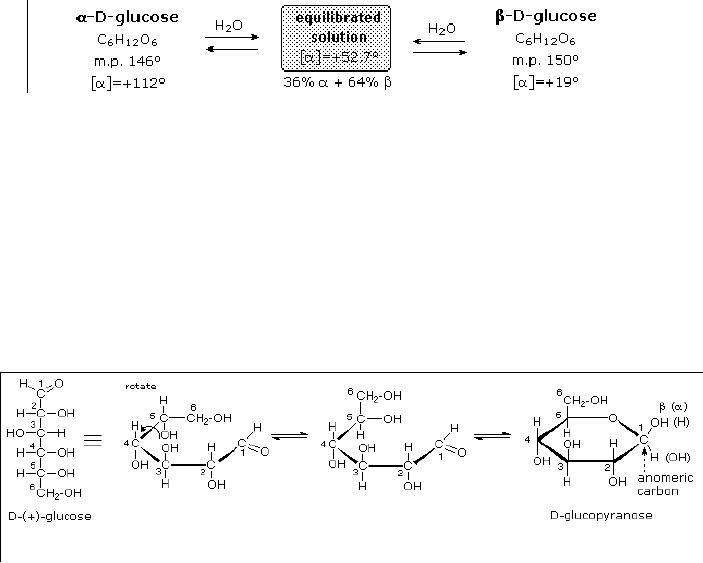
Anomeric
Forms of Glucose
Two
different crystalline forms of
glucose were reported in
1895. Each of these gave all
the
characteristic
reactions of glucose, and when
dissolved in water equilibrated to
the same
mixture.
This equilibration takes place
over a period of many
minutes, and the change in
optical
activity that occurs is
called mutarotation. These
facts are summarized in
the
diagram,
A
simple solution to this
dilemma is achieved by converting
the open aldehyde structure
for
glucose
into a cyclic hemiacetal,
called a glucopyranose, as shown in
the following
diagram.
The
linear aldehyde is tipped on
its side, and rotation about
the C4-C5 bond brings
the C5-
hydroxyl
function close to the aldehyde
carbon. For ease of viewing,
the six-membered
hemiacetal
structure is drawn as a flat
hexagon, but it actually
assumes a chair
conformation.
The
hemiacetal carbon atom (C-1)
becomes a new stereogenic center,
commonly referred to
as
the anomeric carbon, and the
α and β-isomers are called
anomers.
Fig.
15.2. Anomers of
glucose
Cyclic
Forms of Monosaccharides
The
preferred structural form of
many monosaccharides may be that of a
cyclic hemiacetal.
Five
and six-membered rings are
favored over other ring
sizes because of their low
angle and
eclipsing
strain. Cyclic structures of
this kind are termed
furanose (five-membered) or
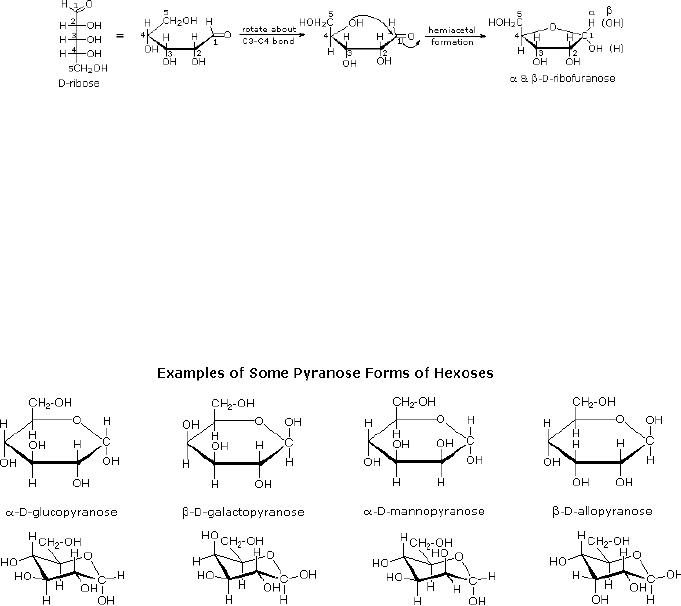
15.8
Carbohydrates
to Chemicals
pyranose
(six-membered), reflecting the
ring size relationship to the
common heterocyclic
compounds
furan and pyran shown on the
right.
Ribose,
an important aldopentose, commonly adopts
a furanose structure, as shown in
the
following
illustration. The upper bond
to this carbon is defined as
beta, the lower bond
then
is
alpha.
Fig.
15.3. Formation of
ribofuranose
The
cyclic pyranose forms of
various monosaccharides are often
drawn in a flat
projection
known
as a Haworth formula, after
the British chemist, Norman
Haworth. These Haworth
formulas
are convenient for
displaying stereochemical relationships,
but do not represent
the
true
shape of the molecules. These
molecules are actually
puckered in a chair
conformation.
Examples
of four typical pyranose
structures are shown below,
both as Haworth
projections
and
as the more representative
chair conformers.
The
size of the cyclic hemiacetal
ring adopted by a given sugar is
not constant, but may
vary
with
substituents and other structural
features. Aldolhexoses usually
form pyranose rings
and
their
pentose homologs tend to
prefer the furanose form,
but there are many
counter
examples.
Synthetic
Strategies in Chemistry
15.9
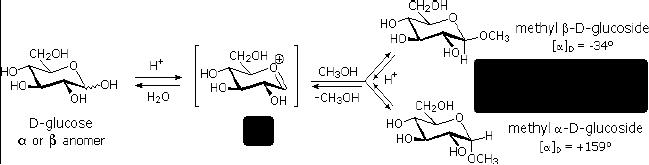
Glycosides
Fig.
15.4. Glycoside
formation
Acetal
derivatives formed when a monosaccharide
reacts with an alcohol in
the presence of
an
acid catalyst are called
glycosides. This reaction is
illustrated for glucose and
methanol in
the
diagram below. In naming of
glycosides, the "ose" suffix
of the sugar name is replaced by
"oside",
and the alcohol group name is placed
first. As is generally true
for most aldols,
glycoside
formation involves the loss of an
equivalent of water. Since
acid-catalyzed
aldolization
is reversible, glycosides may be
hydrolyzed back to their alcohol and
sugar
components
by aqueous acid.
Glycosides
abound in biological systems. By
attaching a sugar moiety to a lipid
or
benzenoid
structure, the solubility and
other properties of the compound
may be changed
substantially.
Because of the important
modifying influence of such
derivatization, numerous
enzyme
systems, known as glycosidases, have
evolved for the attachment
and removal of
sugars
from alcohols, phenols and
amines.
Chemists
refer to the sugar component of
natural glycosides as the
glycon and the
alcohol
component
as the aglycon.Salicin, one of the
oldest herbal remedies
known, was the model
for
the synthetic analgesic aspirin.
Large classes of hydroxylated,
aromatic oxonium
cations
called
anthocyanins provide the
red, purple and blue colors
of many flowers, fruits and
some
vegetables.
Peonin is one example of this
class of natural pigments,
which exhibit
pronounced
pH color dependence. The
oxonium moiety is only stable in
acidic environments
and
the color changes or
disappears when base is
added. The complex changes
that occur
when
wine is fermented and stored are in
part associated with
glycosides of anthocyanins.
15.10
Carbohydrates
to Chemicals
Finally,
amino derivatives of ribose, such as
cytidine play important
roles in biological
phosphorylating
agents, coenzymes and information
transport and storage
materials
Disaccharides
Two
joined monosaccharides are called
disaccharides and represent the
simplest
polysaccharides.
They are composed of two
monosaccharide units bound together by
a
covalent
bond known as a glycosidic
linkage formed via a
dehydration reaction, resulting
in
the
loss of a hydrogen atom from one
monosaccharide and a hydroxyl group from
the other.
Some
examples of disaccharides are;
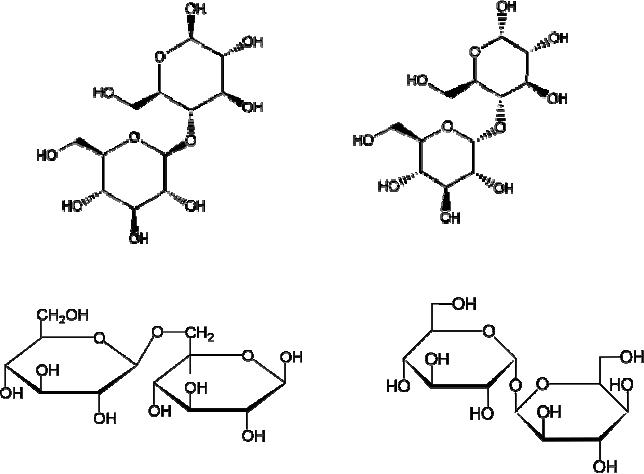
Cellobiose:
4-O-β-D-Glucopyranosyl-D-glucose
Maltose
:
4-O-α-D-Glucopyranosyl-D-glucose
Gentiobiose
:
6-O-β-D-Glucopyranosyl-D-glucose Trehalose
:
α-D-Glucopyranosyl-α-D-glucopyranoside
Cellobiose,
maltose and gentiobiose are hemiacetals
they are all reducing
sugars (oxidized
by
Tollen's reagent). Trehalose, a
disaccharide found in certain mushrooms,
is a bis-acetal,
and
is therefore a non-reducing sugar.
Acid-catalyzed hydrolysis of these
disaccharides
yields
glucose as the only product.
Enzyme-catalyzed hydrolysis is selective
for a specific
Synthetic
Strategies in Chemistry
15.11
glycoside
bond, so an alpha-glycosidase cleaves
maltose and trehalose to glucose,
but does
not
cleave cellobiose or gentiobiose. A
beta-glycosidase has the opposite
activity.
Although
all the disaccharides shown here
are made up of two
glucopyranose rings,
their
properties
differ in interesting ways.
Maltose, sometimes called malt
sugar, comes from
the
hydrolysis
of starch. It is about one third as sweet
as cane sugar (sucrose), is easily
digested
by
humans, and is fermented by yeast.
Cellobiose is obtained by the
hydrolysis of cellulose.
It
has virtually no taste, is
indigestible by humans, and is not
fermented by yeast. Some
bacteria
have beta-glucosidase enzymes that
hydrolyze the glycosidic bonds in
cellobiose and
cellulose.
The presence of such bacteria in
the digestive tracts of cows
and termites permits
these
animals to use cellulose as a
food. Finally, it may be
noted that trehalose has
a
distinctly
sweet taste, but gentiobiose is bitter.
Sucrose, or cane sugar, is our
most commonly
used
sweetening agent. It is a non-reducing disaccharide
composed of glucose and
fructose
joined
at the anomeric carbon of
each by glycoside bonds (one
alpha and one beta).
Polysaccharides
polysaccharides
are large high-molecular
weight molecules constructed by
joining
monosaccharide
units together by glycosidic
bonds. They are sometimes
called glycans.
The
most
important compounds in this
class, cellulose, starch and
glycogen are all polymers
of
glucose.
Cotton fibres are
essentially pure cellulose, and
the wood of bushes and trees
is
about
50% cellulose.
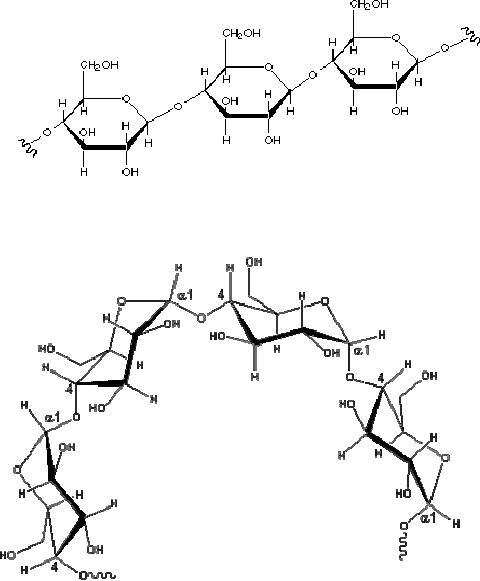
Cellulose
As a
polymer of glucose, cellulose
has the formula (C6H10O5)n where n ranges
from
500 to 5,000, depending on the source of
the polymer. The glucose
units in cellulose
are
linked
in a linear fashion, as shown
below. The beta-glycoside bonds
permit these chains
to
stretch
out, and this conformation is
stabilized by intramolecular hydrogen
bonds. A parallel
orientation
of adjacent chains is also favored by
intermolecular hydrogen bonds.
Although an
individual
hydrogen bond is relatively
weak, many such bonds acting
together can impart
great
stability to certain conformations of
large molecules. Most
animals cannot digest
cellulose
as a food, and in the diets of humans
this part of our vegetable
intake functions as
roughage
and is eliminated largely
unchanged.
Some
animals (the cow and
termites, for example)
harbour intestinal microorganisms
that
breakdown
cellulose into monosaccharide nutrients
by the use of beta-glycosidase
enzymes.
Cellulose
is commonly accompanied by a lower
molecular weight, branched,
amorphous
15.12
Carbohydrates
to Chemicals
polymer
called hemicellulose. In
contrast to cellulose, hemicellulose is
structurally weak and
is
easily hydrolyzed by dilute acid or
base. Also, many enzymes
catalyze its
hydrolysis.
Hemicelluloses
are composed of many
D-pentose sugars, with
xylose being the
major
component.
Mannose and mannuronic acid are
often present, as well as galactose
and
galacturonic
acid.
Fig.
15.5. Cellulose
structure
Fig.
15.6. Representative partial
structure of amylose
Starch
is a
polymer of glucose, found in
roots, rhizomes, seeds, stems,
tubers and corms of
plants,
as microscopic granules having
characteristic shapes and sizes. Most
animals,
including
humans, depend on these
plant starches for
nourishment. The structure of
starch is
Synthetic
Strategies in Chemistry
15.13
more
complex than that of
cellulose. The intact
granules are insoluble in
cold water, but
grinding
or swelling them in warm
water causes them to burst.
The released starch consists
of
two
fractions.
About
20% is a water soluble
material called amylose.
Molecules of amylose are
linear
chains
of several thousand glucose
units joined by alpha C-1 to
C-4 glycoside bonds.
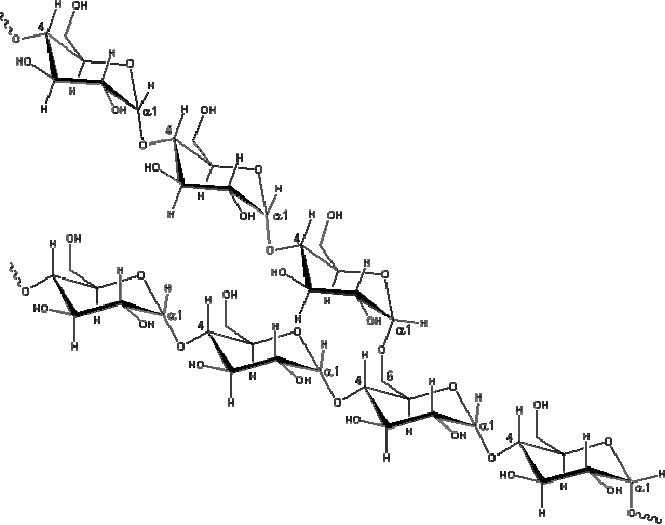
Amylose
solutions are actually dispersions of
hydrated helical micelles.
The majority of the
starch
is a much higher molecular
weight substance, consisting of nearly a
million glucose
units,
and called amylopectin.
Molecules of amylopectin are
branched networks built
from
C-1
to C-4 and C-1 to C-6
glycoside links, and are
essentially water
insoluble.
Fig.
15.7. Representative partial
structure of amylopectin
Glycogen
is a
polysaccharide of glucose (Glc)
which functions as the
primary short term
energy
storage in animal cells.
Glycogen is the analogue of
starch, a less branched
glucose
15.14
Carbohydrates
to Chemicals
polymer
in plants, and is commonly referred to as
animal
starch,
having a similar
structure
to
amylopectin. Glycogen forms an
energy reserve that can be quickly
mobilized to meet a
sudden
need for glucose, but one
that is less compact than
the energy reserves of
triglycerides
(fat).
It is made primarily by the
liver and the muscles, but
can also be made by the
brain.
The
uterus also stores glycogen
during pregnancy to nourish
the embryo.
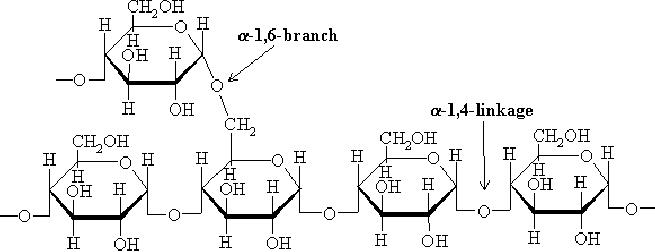
Glycogen
is a highly branched polymer
that is better described as a
dendrimer of about
60,000
glucose residues and has a
molecular weight between
106 and
107 daltons (~4.8
million).
Most of Glc units are
linked by α-1,4 glycosidic
bonds, approximately 1 in 12
Glc
residues
also makes -1,6 glycosidic
bond with a second
Glc,
which results in the
creation of
a
branch. Glycogen does not
possess a reducing
end.
Fig.
15.8. Glycogen
Synthetic
Modification of Cellulose
Cotton,
probably the most useful
natural fiber, is nearly
pure cellulose. Crude
cellulose is
also
available from wood pulp by
dissolving the lignan matrix
surrounding it. These
less
desirable
cellulose sources are widely
used for making paper. In
order to expand the ways
in
which
cellulose can be put to practical
use, chemists have devised
techniques for
preparing
solutions
of cellulose derivatives that can be
spun into fibers, spread
into a film or cast
in
various
solid forms. A key factor in
these transformations are
the three free hydroxyl
groups
on
each glucose unit in the
cellulose chain, --[C6H7O(OH)3]n--.
Esterification of these
Synthetic
Strategies in Chemistry
15.15
functions
leads to polymeric products
having very different
properties compared with
cellulose
itself.
Cellulose
Nitrate,
first prepared over 150
years ago by treating
cellulose with nitric acid,
is
the
arliest synthetic polymer to
see general use. The fully
nitrated compound, --
[C6H7O(ONO2)3]n--,
called guncotton, is explosively
flammable and is a component of
smokeless
powder. Partially nitrated
cellulose is called pyroxylin.
Pyroxylin is soluble in
ether
and at one time was used for
photographic film and lacquers.
The high flammability
of
pyroxylin
caused many tragic cinema
fires during its period of
use. Furthermore,
slow
hydrolysis
of pyroxylin yields nitric
acid, a process that
contributes to the deterioration
of
early
motion picture films in
storage.
Cellulose
Acetate,
--[C6H7O(OAc)3]n--,
is less flammable than
pyroxylin, and has replaced it
in
most applications. It is prepared by
reaction of cellulose with acetic
anhydride and an acid
catalyst.
The properties of the
product vary with the
degree of acetylation. Some
chain
shortening
occurs unavoidably in the
preparations. An acetone solution of
cellulose acetate
may
be forced through a spinnerette to
generate filaments, called
acetate rayon that can
be
woven
into fabrics.
Viscose
Rayon ,
is prepared by formation of an alkali
soluble xanthate derivative
that can be
spun
into a fiber that reforms
the cellulose polymer by acid
quenching. The following
general
equation
illustrates these transformations.
The product fiber is called
viscose rayon.
H3O(+)
NaOH
RO-CS2(-) Na(+)
RO(-) Na(+) +
S=C=S
ROH
ROH
cellulose
viscose
solution
rayon
Catalytic
conversion of carbohydrates
Carbohydrates
are the main source of
renewables used for the
production of bio-based
products.
Sucrose and starch are the
major sources. Polysaccharides such as
inulin are
gaining
importance as a source of fructose. Two
carbohydrates of animal origin, lactose
and
chitin
are also used
commercially.
Multistep
reactions carried out by cascade
catalysis without intermediate
product
recovery
decrease operating time and
may reduce considerably the
amount of waste
produced.
For example, sorbitol can be
obtained in one-pot reaction
from starch-derived

15.16
Carbohydrates
to Chemicals
polysaccharides
using ruthenium supported on
acidic Y-zeolite. The acidic
sites of the zeolite
catalyze
the polysaccharide hydrolysis
yielding transiently glucose,
which is hydrogenated to
sorbitol
on ruthenium. Similarly,
2,5-Furanedicarboxylic acid, a potential
substitute for
terephtalic
acid is obtained in one-pot reaction
over a bifunctional acidic and
redox catalyst
consisting
of cobalt acetylacetonate encapsulated in
solgel silica. In the
three former
examples,
the reactions steps took place on
heterogeneous catalysts. However,
cascade
catalysis
without recovery of intermediate
products may involve
enzymatic catalysis,
homogeneous
catalysis and heterogeneous catalysis.
Combination of enzymatic and
chemical
steps
can give a better
yield.
Hydrogenation
of glucose and
derivatives
Glucose
issued from starch or
sucrose hydrolysis is hydrogenated to
sorbitol , a commodity
product
used in food, pharmaceutical and
chemical industries as well as an
additive in many
end-products.Mannitol
and gluconic acid are the
main by-products of this
reaction.
Fig.
15.9.
Hydrogenation of glucose
Catalysts
allowing a 100% conversion and
99% selectivity are
required. Also, they should
be
stable
after many recycling
operations or for extended
period of time on stream in
continuous
reactor.
Most of the industrial
production is still conducted
batch-wise on Raney
nickel
catalysts
promoted with electropositive
metal atoms such as molybdenum and
chromium ,but
because
of the risk of nickel or
metallic promoter leaching,
they tend to be replaced by
carbon
supported ruthenium catalysts
which are also more active.
However, active
carbon
Synthetic
Strategies in Chemistry
15.17
powders
are difficult to handle and
recycle in batch operation,
therefore a continuous
process
with
formed carbon support are
desirable.
Hydrogenation
of glucose to sorbitol was achieved on
ruthenium catalysts supported
on
activated
carbon cloths (ACC) obtained
by carbonization and CO2 activation of woven
rayon
.Catalyst
0.9 wt.% Ru/ACC was loaded
with ruthenium by cationic
exchange or anionic
adsorption
both giving an homogeneous distribution
of 2 nm ruthenium particles in
carbon
fibers.
The ACC was clamped on a
support fitting along the
autoclave walls thus
allowing an
easy
recycling of the catalyst since,
unlike catalysts in powder
form, no filtration are
required
and
there is no attrition or
leaching.
There
is a great interest to convert C6
carbohydrates available in large
supply from starch
or
sucrose into C5 and C4 polyols
that are little present in
biomass and find many
applications
in food and non-food products.
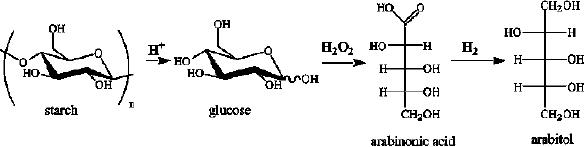
Glucose can be converted to arabitol by
an
oxidative
decarboxylation of glucose to arabinonic
acid followed by hydrogenation to
arabitol.The
main drawback of this
reaction is the formation of
deoxy products.
Aqueous
solutions
(20 wt. %) of arabinonic acid
were hydrogenated on Rucatalysts in
batch reactor.
The
selectivity was enhanced by adding small
amounts of anthraquinone-2-sulfonate
(A2S),
which
decreased the formation of
deoxy by-products.
Fig.
15.10. Oxidative decarboxylation of
glucose
Dehydroxylation
of carbohydrates
Deoxyhexitols
consisting of C6 diols, triols, and
tetrols are well suited to
replace polyols
derived
from petrochemistry for
applications in polyester and
polyurethane manufacture.
Sorbitol
was taken as model molecule to
study the hydrogenolysis to
C4C6 products. To
improve
the selectivity to deoxyhexitols,
catalysts and reaction temperature
were optimized
to
favor the rupture of COH bonds
(dehydroxylation reactions) rather
than CC bond
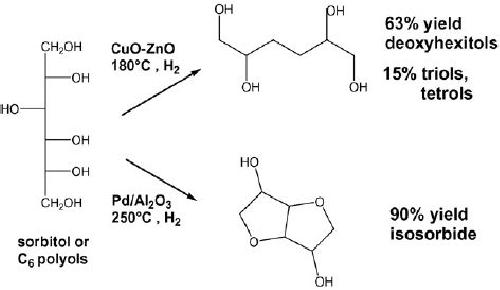
15.18
Carbohydrates
to Chemicals
rupture.
Copper-based catalysts, which
have a low activity for
hydrogenolysis of CC
bonds,
were
employed to treat 20 wt.%
aqueous sorbitol solutions in
the temperature range of
180
240
8C. In contrast, operating in
the presence of palladium
catalysts at 250 0C
under 80 bar
of
hydrogen pressure, cyclodehydration
reactions of sorbitol and mannitol
occurred with
formation
of cyclic ethers: isosorbide,
2,5-anhydromannitol, 2,5-anhydroiditol, and
1,4-
anhydrosorbitol
.
Catalytic
oxidation of mono- and
di-saccharides
Oxidation
reactions are widely used
for upgrading carbohydrates to
varieties of high
added
value
chemicals used in detergents or
pharmaceuticals (Vitamin C). To replace
the non-green
hypochlorite
agent by environmentaly friendly reagents,
the catalytic system was
improved.
Oxidation
reactions with H2O2 mediated by metal phthalocyanine
catalysts have also
proved
very
efficient to oxidize various
carbohydrates including the
oxidation of insoluble
substrates
such
as native starch.
Fig.
15.11.
Hydrogenolysis
of sorbitol to C6 polyols
Glucose
oxidation to gluconic acid, a biodegradable
chelating agent and an intermediate
in
food
and pharmaceutical industry, was achieved
with air oxidation in the
presence of
palladium
catalysts. Unpromoted palladium
catalysts were active in
glucose oxidation,
but
the
rate of reaction was low because of
the over-oxidation of Pd-surface, and
side oxidation
reactions
decreased the selectivity.
Using PdBi/C catalysts (5
wt.% Pd, Bi/Pd =
0.1)
Synthetic
Strategies in Chemistry
15.19
prepared
by deposition of bismuth on the
surface of 12 nm palladium
particles, the rate of
glucose
oxidation to gluconate was 20 times
higher and the selectivity at near
total
conversion
was high on the fresh and
recycled catalysts. Bismuth was
assumed to act as a
cocatalyst
protecting palladium from
over-oxidation because of its
stronger affinity for
oxygen.
The metal-catalyzed oxidation gave
comparable selectivity and higher
productivity
than
enzymatic glucose
oxidation.
Catalytic
Conversion of Polysaccharides
Polysaccharides
are widely available
renewable polymers but it is
difficult to find cost
effective
process to convert them to
valuable end-products. Due to
its large availability
and
low
cost, native starch has
been used for a long
time in the preparation of
different end-
products.
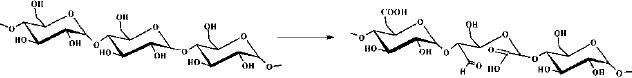
To
meet specific hydrophilic properties
native starch has been
either modified by
oxidation
or
by grafting hydrocarbon chains
.Hydrophilic
starch obtained by partial
oxidation is widely
used
in paper and textile industries and can
be potentially applied in a variety of
applications,
e.g.,
for the preparation of
paints, cosmetics, and super absorbents. The
oxidation occurs at
the
C6 primary hydroxyl group or at
the vicinal diols on C2 and C3
involving a cleavage of
the
C2C3 bond to give
carbonyl and carboxyl
functions.
Fig.
15.12. Hydrophilic starch
obtained by partial
oxidation
Several
transition metal catalysts
based on Fe, Cu or W salts (0.010.1
mol%) have been
proposed
to activate H2O2,
which is a well-suited oxidant
from an environmental and
economical
point of view. However, the
concentration of metal ions was
quite high and
heavy
metals were retained by the
carboxyl functions of oxidized
starch, which has
good
complexing
properties. Efficient catalytic
methods for native starch
oxidation withH2O2 in
the
presence of iron tetrasulfophthalocyanine
(FePcS) were proposed .Oxidation
of starch
aqueous
suspension in the presence of iron
phthalocyanine gives both
carboxylic and
carbonyl
groups.
15.20
Carbohydrates
to Chemicals
To
prepare more hydrophobic
starches for specific
applications, partial substitution of
starch
with
acetate, hydroxypropyl, alkylsiliconate
or fatty-acid ester groups
were described in the
literature.
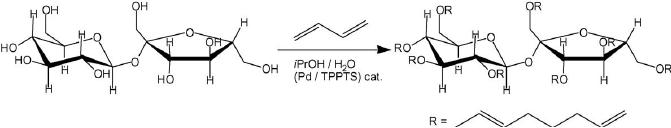
This can also be achieved by grafting
octadienyl chains by
butadiene
telomerization.
Fig.
15.13. Grafting of octadienyl
chains on sucrose through
butadiene telomerization
The
reaction was first conducted
with success on sucrose. The
degree of substitution
(DS)
obtained
was controlled by the reaction
time. Thus under standard
conditions (0.05%
Pd(OAc)2/TPPTS,
NaOH (1N)/iPrOH (5/1), 50
8C) the DS was 0.5 and 5
after 14 and 64 h
reaction
time, respectively. The
octadienyl chains were
hydrogenated quantitatively in
the
presence
of 0.8 wt.% [RhCl(TPPTS)3]
catalyst in H2O/EtOH (50/10)
mixture yielding a
very
good
biodegradable surfactant.
REFERENCES:
1.
www.wikipedia.org
2.
www.
users.rcn.com/jkimball.ma.ultranet/BiologyPages/C/Carbohydrates
3.
www.biochemweb.org/fenteany/teaching/452/Carbohydrates.pdf
4.
P.A. Jacobs, H. Hinnekens, EP 0 329 923
for Synfina-Oleofina
5.
M.L. Ribeiro, U. Schuchardt, Catal.
Commun.,
4 (2003) 83
6.
A. Perrard, P. Gallezot, in: J. Sowa
(Ed.), Catalysis
of Organic Reactions,
Taylor
& Francis, New York,
2005, p. 53.
7.
L. Fabre, P. Gallezot, A. Perrard, J.
Catal., 208
(2002) 247
8.
B. Blanc, A. Bourrel, P. Gallezot, T.
Haas, P. Taylor, Green
Chem., 89
(2000)
9.
M. Besson, F. Lahmer, P. Gallezot, P.
Fuertes, G. Fle`che, J.
Catal. ,
152 (1999) 116
Synthetic
Strategies in Chemistry
15.21
10.
A. Sorokin, S. Kachkarova-Sorokina, C.
Donze�, C. Pinel, P. Gallezot,
Top.
Catal.,
27 (2004)
67
11.
A. Sorokin, S. Sorokina, P. Gallezot,
Chem.
Commun.,
2004, 2844
12.
C. Donze�, C. Pinel, P. Gallezot, P.
Taylor, Adv.
Synth. Catal., 344
(2002) 906
Table of Contents:
- INTRODUCTION TO SYNTHETIC STRATEGIES IN CHEMISTRY:POROUS MATERIALS
- SYNTHETIC METHODS BASED ON ACTIVATING THE REACTANT:HALOGENATION OF BENZENE
- METHODS BASED ON ACTIVATING THE REACTING SUBSTANCE:Experimental method
- SYNTHESIS OF MATERIALS BASED ON SOLUBILITY PRINCIPLE
- SOL-GEL TECHNIQUES:DEFINITIONS, GENERAL MECHANISM, INORGANIC ROUTE
- TEMPLATE BASED SYNTHESISSynthesis, Mechanism and Pathway
- MICROEMULSION TECHNIQUES:Significance of Packing Parameter
- SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS
- NEWER SYNTHETIC STRATERGIES FOR NANOMATERIALS:Nanostructured Materials
- THE ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY:The Holy Bible
- ELECTROCHEMICAL SYNTHESIS:FEATURES OF ELECTROCHEMICAL SYNTHESIS
- NEWER REACTIONS AND PROCEDURES: CATALYTIC AND NONCATALYTIC
- SYNTHETIC STRATEGIES - FROM LABORATORY TO INDUSTRY
- SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice
- CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES
- SOME CONCEPTUAL DEVELOPMENTS IN SYNTHESIS IN CHEMISTRY
- COMPUTATIONAL BASICS UNDERLYING SYNTHETIC STRATEGIES