 |
DEVELOPING ISO QMS FOR CERTIFICATION:Process approach |
| << DESIGNING ORGANIZATIONS FOR QUALITY:Customer focus, Leadership |
| ISO 9001(2000) QMS MANAGEMENT RESPONSIBILITY:Issues to be Considered >> |

Total
Quality Management
MGT510
VU
Lesson
# 22
DEVELOPING
ISO QMS FOR
CERTIFICATION
DEVELOPMENT,
IMPLEMENTATION AND
REGISTRATION
The
ISO 9000 standards
originally were intended to be advisory
in nature and to be used for
two-party
contractual
situations (between a customer and
supplier) and for internal
auditing. However,
they
quickly
evolved into criteria for
companies who wished to
"certify" their quality
management or
achieve
"registration" through a third-party
auditor, usually a laboratory or
some other
accreditation
agency
(called a registrar). This
process began in the United
Kingdom. Rather than a supplier
being
audited
for compliance to the standards by
each customer, the registrar certifies
the company, and this
certification
is accepted by all of the supplier's
customers.
The
registration process includes document
review by the registrar of the
quality system documents
or
quality
manual; pre-assessment, which
identifies potential noncompliance in the
quality system or in the
documentation;
assessment by a team of two or three
auditors of the quality system
and its
documentation;
and surveillance, or periodic re-audits to
verify conformity with the
practices and
systems
registered. During the assessment,
auditors might ask such
question as (using
management
responsibility
as an example): Does a documented policy
on quality exist? Have job descriptions
for
people
who manage or perform work
affecting quality been
documented? Are descriptions of
functions
that
affect quality been
documented? Are descriptions of functions
that affect quality
available? Has
management
designated a person or group with the
authority to prevent nonconformities in
products,
identify
and record quality problems, and recommend solutions?
What means are used to
verify the
solutions?
Re-certification
is required every three years.
Individual sites not
entire companies must
achieve
registration
individually. All costs are
borne by the applicant, so the process
can be quite
expensive.
Perspectives
on ISO 9000:2000
ISO
9000 provides a set of good
basic practices for
initiating a quality system, and is an
excellent
starting
point for companies with no
formal quality assurance
program. In fact, it provides
detailed
guidance
on process and product control. Thus,
for companies in the early
stages of developing a
quality
program,
the standards enforce the discipline of
control that is necessary
before they can
seriously
pursue
continuous improvement. The requirements
of periodic audits reinforce the stated
quality system
until
it becomes ingrained in the company.
Thus, using ISO 9000 as a
basis for a quality system
can
improve
discipline, process, productivity,
decrease costs, and increase
customer satisfaction.
ISO
9000 as a Quality Journey
and not as a Destination
How
to get started is always an issue
for organizations just
beginning their total
quality journey.
The
ISO 9000 development effort
will benefit by having the
following components: an
executive-level
steering
committee, a vision with the
guiding principles, a set of
broad objectives, baselines
on
employee
and customer satisfaction, an
objective view of the organization's
strengths and weaknesses,
and
an indication of which employees at all
levels can be counted on for support
during the
implementation.
In addition, the organization will have a
well thought out means of
communicating
with
employees and all other stakeholders to keep them
apprised of the changes taking place, why
they
are
happening, and what they
will mea to everyone.
The
development and implementation of an
organization's quality management
system is influenced by
its
varying needs, its
particular objectives, the products it
provides, and the processes it
employs. It is
not
the purpose of this ISO to
imply uniformity of quality
management systems.
81

Total
Quality Management
MGT510
VU
The
selection of the appropriate quality
related processes described in this
ISO Standard and the extent
to
which these processes are
adopted and applied by an organization
depends upon factors such as
its
size
and structure, the market being
served and the resources
available.
The
purpose of an organization
is:
a)
To
identify and meet the needs
and expectations of its customers and
other interested parties
(i.e.
employees, suppliers, owners, society), to achieve
competitive advantage, and to do this
in
an
effective and efficient manner;
b)
To
achieve, maintain, and improve overall
organizational performance and
capabilities.
The
application of quality management
principles not only provides
direct benefits but also
makes an
important
contribution to managing costs and risks.
Benefit, cost and risk
considerations are important
for
the organization, its customers and
other interested parties. These considerations on
overall
performance
may impact on the
following:
a}
Revenue
(turnover), profits and market
share; these may be
increased by such aspects
as
leadership,
increased efficiency, improved
employee performance, and employee
and customer
satisfaction;
b)
Costs
due to resources needed for
business; inadequate resource funding is
likely to cause
losses
and
be a competitive disadvantage through the
sale of deficient products.
Process
approach
The
ISO-9001(2000) Standard encourages the
adoption of a process approach to quality
management.
Any
activity which receives
inputs and converts them to outputs can be considered
as a process. For
organizations
to function effectively, they have to
identify and manage numerous
inter-linked processes.
Often
the output from one process
will directly form the input
into the next process. The
systematic
identification
and management of the processes employed
by an organization, and the
interactions
between
such processes, may be
referred to as the 'process
approach'.
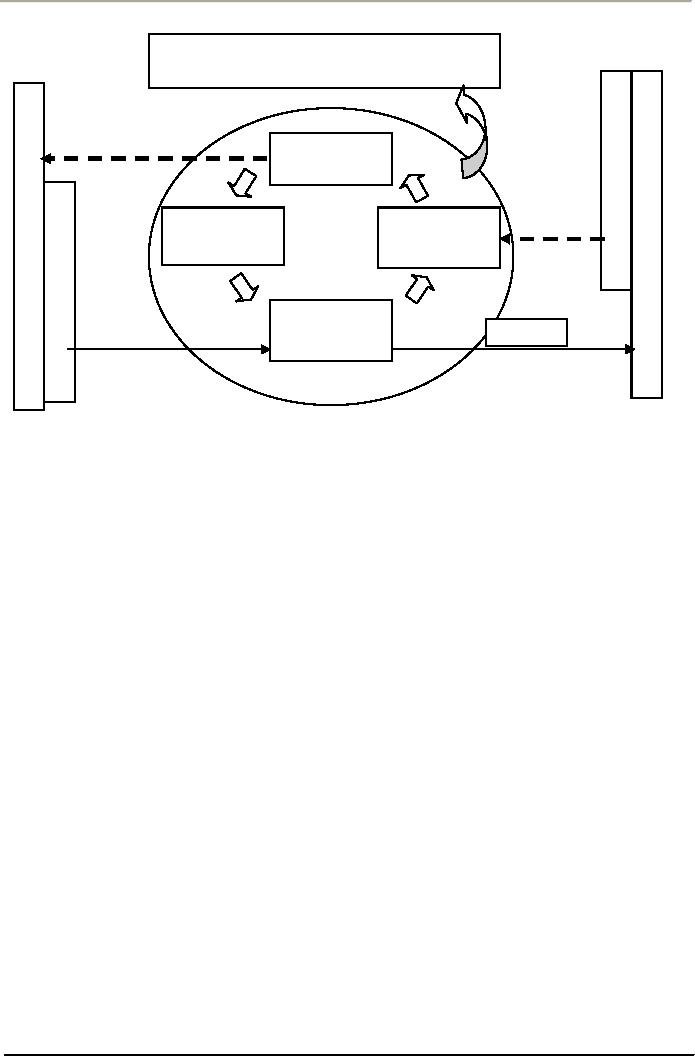
Following
Figure is a conceptual illustration of
one of the process approach. The
model recognizes that
customers
and other interested parties play a
significant role in defining
inputs. Monitoring the
satisfaction
of customers and other interested parties is
necessary to evaluate and validate
whether the
requirements
of customers and other interested parties have
been met. This model does
not reflect
processes
at a detailed level, but
covers all the contents of the
ISO Standard.
The
purpose of ISO 9001 is to
define the minimum Quality
Management System requirements
needed
to
achieve customer satisfaction by meeting
specified product requirements.
Compliance with ISO
9001
may
be used by an organization to demonstrate
its capability to meet
customer requirements.
82
Total
Quality Management
MGT510
VU
CONTINUAL
IMPROVEMENT OF THE
QUALITY
MANGEMENT SYSTEM
S
I
I
a
N
N
t
T
T
i
E
Management
`
E
s
R
Responsibility
R
f
E
E
a
S
R
S
c
T
e
T
Resource
Measurement,
t
E
q
E
analysis
Management
i
D
u
D
improvement
o
i
P
n
r
P
A
e
A
R
Product
m
Input
Output
R
T
realization
Product
e
T
I
n
I
E
t
E
S
s
S
Terms
and definitions
For
the purposes of this ISO
9001(2000) International Standard, the
terms and definitions given in
ISO
9000:2000,
are applied in following
way.
Supplier
--------- >Organization ----------
>Customer (Interested
Parties)
The
term "organization" replaces the
previously used term
"supplier", to mean the unit
to which
this
International Standard applies. The
term "supplier" is now used
instead of the previous
term
"subcontract".
The changes have been
introduced to reflect the vocabulary
used by
organizations.
Product
as a result of a Process
There
are four agreed generic
product categories as per
ISO-9000:
·
Hardware,
·
Software,
·
Services,
·
Processed
materials.
Most
products are combinations of some of the
four generic product categories.
Whether
the combined product is then
called hardware, processed material,
software or service depends
on
the dominant element.
Following
is one of the model methodology which
can be used to develop the
documentation for QMS
and
to get ready for certification by
implementing clause by clause
requirements (in the box) as given
in
ISO
9001(2000) as the minimum requirement
for certification and one can
also take help from
the
guidelines
given in ISO-9004(2000) as presented
below after the boxes.
83

Total
Quality Management
MGT510
VU
ISO
Quality Management System
Requirements/Guidelines
ISO
9001:2000 - Quality management systems
-.Requirements
General
requirements
The
organization shall establish, document,
implement, maintain a quality
management and continually
improve
its effectiveness in accordance with the
requirement of this International
Standard.
The
organization shall:
a)
Identify
the processes needed for the
quality management system and
their application
throughout
the organization (see
1.2)
b)
Determine
the sequence and interaction of these
processes;
c)
Determine
criteria and methods needed to
ensure that both the
operation and control of the
processes
are effective,
d)
Ensure
the availability of resources and
information necessary to support the
operation and
monitoring
of these processes;
e)
Measure,
monitor and analyze these
processes, and
f)
Implement
action necessary to achieve planned
results and continual improvement
these
processes.
These
processes shall be managed by the
organization in accordance with the
requirements of this
International
Standard.
Where
an organization chooses to outsource
any process that affects
product conformity
with
requirements,
the organization shall ensure
control over such
processes.
NOTE
Processes
needed for the quality
management system referred to above
should include
processes
for
management activities, provision of
resources, product realization and
measurement.
Quality
management system guidelines
Managing
systems and
processes
Leading
and operating an organization successfully requires
managing it in a systematic and
visible
manner.
Success should result from
implementing and maintaining a management
system that is
designed
to continually improve performance by
addressing the needs of all interested
parties.
Managing
an organization encompasses quality
management, among other management
disciplines.
The
quality management system of an
organization is an important part of the
overall management
system.
Organizations should define
their systems and the processes
contained within them to enable
the
systems
and processes to be clearly understood,
managed and improved. Management
should ensure
effective
operation and control of processes and
the measures and data used to
determine satisfactory
performance.
The management of the organization
should closely monitor the movement
toward
performance
improvement. The activities and
processes that can lead to
performance improvement
should
be described and defined by the
management. In general, to fulfill the requirements of
ISO
standard;
First,
Company should state
(write/document) what do they
want to do
Second,
do the work as was stated and
documented
Third,
check them, weather the work is being
carried out as was stated.
See if there are any gaps
.
Fourth,
Show and prove to an external
auditor that work is really
being done as was stated in the
first
place.
84

Total
Quality Management
MGT510
VU
So
what in terms of documentation
required is a manual altogether in one
volume to be called as
ISO
9000
QMS Manual or can be
separated into following
manuals:
1.
Quality
Policy Manual
2.
Quality
Procedures Manual
3.
Quality
Work Instructions
4.
Quality
Records Manual or Quality Data
Collection Manual
Documentation
and records may be in any
form or in any media suitable
for the needs of the
organization.
Requirements
for documentation and records
may arise from
·
contractual
requirements from the customer or other
interested parties,
·
acceptance
of international, national, regional
and industry sector
standards,
·
relevant
statutory and regulatory requirements,
or
·
decisions
by the organization.
ISO
9001:2000 Quality management
systems -Requirements
Documentation
Requirements
General
The
quality management system
documentation shall
include:
a)
Documented
statements of a quality policy and
quality objectives
b)
A
quality manual
c)
Documented
procedures required by this
International Standard;
d)
Documents
needed by the organization to ensure the
effective planning, operation and
control
Use
of quality management principles
ISO
9001:2000 Quality management
systems -Requirements
Documentation
Requirements
Quality
Manual
The
organization shall establish and maintain
a quality manual that
includes
a)
The
scope of the quality management
system, including details of and
justification for any
exclusions
(see 1.2),
b)
The
documented procedures established for the
quality management system, or reference
to
them,
and
c)
A
description of the interaction between the
processes of the quality management
system.
Control
of Documents
Documents
required by the quality management
system shall be controlled.
Records are a special
type
of
document and shall be controlled
according to the requirements given in
4.2.4.
A
documented procedure shall be established to define
the controls needed.
a)
to
approve documents for
adequacy prior to
issue,
b)
to
review and update as necessary and
re-approve documents,
85

Total
Quality Management
MGT510
VU
c)
to
ensure that changes and the
current revision status of
documents are
identified,
d)
to
ensure that relevant versions of
applicable documents are
available at points of
use,
e)
to
ensure that documents remain
legible and readily
identifiable,
f)
to
ensure that documents of
external origin are
identified and their distribution
controlled,
and
g)
to
prevent the unintended use of obsolete
documents, and to apply suitable
identification to
them
if they are retained for
any purpose.
Control
of Records
Records
shall be established and maintained to
provide evidence o conformity to requirements and
of
the
effective operation of the quality
management system. Records
shall remain legible,
readily
identifiable
and retrievable. A documented procedure shall be
established to define the controls
needed
for
the identification, storage, protection,
retrieval, retention time and
disposition of records.
Documentation
and Records
Management
should define the documentation
needed to support the quality management
system. The
nature
and extent of the documentation should
support the needs of tile organization.
The defined
documentation
should provide for
implementation, maintenance and improvement of the
system.
This
documentation typically
includes
·
policy
documents including the quality
manual
·
documentation
for control of
processes,
·
work
instructions for defined
tasks,
·
standard
formats for collection and reporting of
data and
·
quality
records.
The
primary purpose of quality
documentation is to express the
quality policy and to
describe the
quality
management system. This
documentation serves as the basis
for implementation
and
maintenance
of the system. Suitable documentation
should be available to achieve the
effective
operation
of the quality management
system.
Documentation
control should be defined and
implemented to ensure that correct
documents arc used.
All
obsolete documents should be promptly
removed from all points of
issue and use, or
otherwise
prevented
from unintended use.
Documents
to be retained, and records of quality
performance, should be controlled,
maintained and
protected.
The
organization should ensure
that sufficient records be
maintained to demonstrate conformance
to
requirements
and verify effective operation of the
quality management system.
These records can
also
provide
know1edge for maintenance and improvement
of the quality management
system.
Quality
records should be analyzed to
provide inputs for
corrective and preventive action, and
process
improvements.
Analysis of records may also
provide information for use
in the improvement of the
Quality
management system.
86
Table of Contents:
- OVERVIEW OF QUALITY MANAGEMENT:PROFESSIONAL MANAGERIAL ERA (1950)
- TOTAL QUALITY MANAGEMENT AND TOTAL ORGANIZATION EXCELLENCE:Measurement
- INTEGRATING PEOPLE AND PERFORMANCE THROUGH QUALITY MANAGEMENT
- FUNDAMENTALS OF TOTAL QUALITY AND RATERS VIEW:The Concept of Quality
- TOTAL QUALITY MANAGEMENT AND GLOBAL COMPETITIVE ADVANTAGE:Customer Focus
- TOTAL QUALITY MANAGEMENT AND PLANNING FOR QUALITY AT OFFICE
- LEADERS IN QUALITY REVOLUTION AND DEFINING FOR QUALITY:User-Based
- TAGUCHI LOSS FUNCTION AND QUALITY MANAGEMENT
- WTO, SHIFTING FOCUS OF CORPORATE CULTURE AND ORGANIZATIONAL MODEL OF MANAGEMENT
- HISTORY OF QUALITY MANAGEMENT PARADIGMS
- DEFINING QUALITY, QUALITY MANAGEMENT AND LINKS WITH PROFITABILITY
- LEARNING ABOUT QUALITY AND APPROACHES FROM QUALITY PHILOSOPHIES
- TOTAL QUALITY MANAGEMENT THEORIES EDWARD DEMING’S SYSTEM OF PROFOUND KNOWLEDGE
- DEMING’S PHILOSOPHY AND 14 POINTS FOR MANAGEMENT:The cost of quality
- DEMING CYCLE AND QUALITY TRILOGY:Juran’s Three Basic Steps to Progress
- JURAN AND CROSBY ON QUALITY AND QUALITY IS FREE:Quality Planning
- CROSBY’S CONCEPT OF COST OF QUALITY:Cost of Quality Attitude
- COSTS OF QUALITY AND RETURN ON QUALITY:Total Quality Costs
- OVERVIEW OF TOTAL QUALITY APPROACHES:The Future of Quality Management
- BUSINESS EXCELLENCE MODELS:Excellence in all functions
- DESIGNING ORGANIZATIONS FOR QUALITY:Customer focus, Leadership
- DEVELOPING ISO QMS FOR CERTIFICATION:Process approach
- ISO 9001(2000) QMS MANAGEMENT RESPONSIBILITY:Issues to be Considered
- ISO 9001(2000) QMS (CLAUSE # 6) RESOURCES MANAGEMENT:Training and Awareness
- ISO 9001(2000) (CLAUSE # 7) PRODUCT REALIZATION AND CUSTOMER RELATED PROCESSES
- ISO 9001(2000) QMS (CLAUSE # 7) CONTROL OF PRODUCTION AND SERVICES
- ISO 9001(2000) QMS (CLAUSE # 8) MEASUREMENT, ANALYSIS, AND IMPROVEMENT
- QUALITY IN SOFTWARE SECTOR AND MATURITY LEVELS:Structure of CMM
- INSTALLING AN ISO -9001 QM SYSTEM:Implementation, Audit and Registration
- CREATING BUSINESS EXCELLENCE:Elements of a Total Quality Culture
- CREATING QUALITY AT STRATEGIC, TACTICAL AND OPERATIONAL LEVEL
- BIG Q AND SMALL q LEADERSHIP FOR QUALITY:The roles of a Quality Leader
- STRATEGIC PLANNING FOR QUALITY AND ADVANCED QUALITY MANAGEMENT TOOLS
- HOSHIN KANRI AND STRATEGIC POLICY DEPLOYMENT:Senior Management
- QUALITY FUNCTION DEPLOYMENT (QFD) AND OTHER TOOLS FOR IMPLEMENTATION
- BASIC SQC IMPROVEMENT TOOLS:TOTAL QUALITY TOOLS DEFINED
- HOW QUALITY IS IMPLEMENTED? A DIALOGUE WITH A QUALITY MANAGER!
- CAUSE AND EFFECT DIAGRAM AND OTHER TOOLS OF QUALITY:Control Charts
- STATISTICAL PROCESS CONTROL (SPC) FOR CONTINUAL QUALITY IMPROVEMENT
- STATISTICAL PROCESS CONTROL….CONTD:Control Charts
- BUILDING QUALITY THROUGH SPC:Types of Data, Defining Process Capability
- AN INTERVIEW SESSION WITH OFFICERS OF A CMMI LEVEL 5 QUALITY IT PAKISTANI COMPANY
- TEAMWORK CULTURE FOR TQM:Steering Committees, Natural Work Teams
- UNDERSTANDING EMPOWERMENT FOR TQ AND CUSTOMER-SUPPLIER RELATIONSHIP
- CSR, INNOVATION, KNOWLEDGE MANAGEMENT AND INTRODUCING LEARNING ORGANIZATION