 |
SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice |
| << SYNTHETIC STRATEGIES - FROM LABORATORY TO INDUSTRY |
| CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES >> |
Chapter
- 14
SYNTHESIS
OF CHEMICALS FROM CARBON DIOXIDE
T.
M. Sankaranarayanan
INTRODUCTION
Carbon
dioxide is an atmospheric gas
contains one carbon and two
oxygen atoms. It is
well
known chemical compound and
its chemical formula
CO2.
Dry ice
is
nothing but
solid-state
carbon dioxide. It was one of the
first gases to be described as a
substance
distinct
from air.
In
the 17th century, the
Flemish chemist Jan Baptist
van Helmont observed that
when
he
burnt charcoal in a closed vessel, the
mass of the resulting ash
was much less than
that
of
the original charcoal. His
interpretation was that the rest of
the charcoal had been
transmuted
into an invisible substance he
termed a "gas" or "wild
spirit" (spiritus
sylvestre). The
Scottish physician Joseph
Black studied some of the
properties more
thoroughly
in the 1750's. He found that
limestone (calcium carbonate) could be
heated or
treated
with acids to yield a gas as
he termed "fixed air." He observed
that the fixed
air
was
denser than air and did
not support either flame or
animal life. He also found
that it
would,
when bubbled through an
aqueous solution of lime
(calcium hydroxide),
precipitate
calcium carbonate, and used this
phenomenon to demonstrate that
carbon
dioxide
is produced by animal respiration and
microbial fermentation. In 1772,
Joseph
Priestley
used carbon dioxide produced
from the action of sulfuric
acid on limestone to
prepare
soda water, the first
known instance of an artificially
carbonated beverage. CO2
was
first liquefied (at elevated
pressures) in 1823 by Humphrey Davy and
Michael
Faraday.
The earliest description of
solid carbon dioxide was
given by Charles
Thilorier,
who
in 1834 opened a pressurized container of
liquid carbon dioxide, only
to find that the
cooling
produced by the rapid
evaporation of the liquid
yielded a "snow" of solid
CO2.
Most
of the combustion of organic
matter, volcanic out gassing
and respiration
processes
of living aerobic organisms are the
main sources of the carbon
dioxide. Various
microorganisms
from fermentation and cellular
respiration also produce it.
During the
photosynthesis
plants take in the carbon
dioxide and using both the
carbon and oxygen to
form
carbohydrates. In addition, plants also
release oxygen to the atmosphere,
which is
subsequently
used for respiration by
heterotrophic organisms, forming a
cycle. It is
14.2
Synthesis
of Chemicals from Carbon
dioxide
present
in the earth's atmosphere at a low
concentration and acts as a greenhouse
gas. It
is
a major component of the
cycle.
Green
house effect is nothing but an
increase in the absorption of
radiation energy from
sun
caused by the existence of
gases in the earth atmosphere.
Because of this
absorption
the
earth atmospheric temperature is
raising which is called
"global warming". Some
of
the
gases like CO2,
CH4, O3, CFCs and H2O
vapors are called "green
house gases".
Mainly
these gases are responsible
for this absorption.
CO2 being an important
member
of
these gases is responsible
for many climatic changes
demonstrating the importance
of
CO2 content in atmosphere. Because of
mounting of Industrial Revolution,
the Percentage
of
carbon dioxide in the earth
atmosphere is increasing virtually at the
rate of 1% per
annum;
from 250ppm of the
pre-industrial period to a present level
of 400ppm (315ppm
in
1958, 340ppm in1984).
Because of this global
warming the snow cover in
the northern
hemisphere
and floating ice in the artic
ocean have decreased
considerably. Moreover,
globally
sea water level increased up
to 8 inches in the last
decade. There is an increase
in
worldwide
precipitation by one percent. There is
abnormal rainfall through
out the world.
Unfortunately,
greenhouse gases are likely to
increase the rate of climate
changes.
CARBON
DIOXIDE - CHEMICAL AND PHYSICAL PROPERTIES
1)
Carbon dioxide is a colorless
gas.
2)
When inhaled at high
concentrations (a dangerous activity
because of the
associated
asphyxiation risk), it produces a sour
taste in the mouth and a
stinging
sensation
in the nose and
throat.
3)
These kind of effects result
from the gas dissolving in
the mucous membranes
and
saliva, forming a weak
solution of carbonic
acid.
4)
Its density at 25 °C is 1.98 kg
m-3,
about 1.5 times that of
air.
5)
It has no electrical dipole. As it is
fully oxidized, it is not very
reactive and, in
particular,
not flammable.
6)
At temperatures below -78 °C, carbon
dioxide condenses into a
white solid called
dry
ice. Liquid carbon dioxide
forms only at pressures above
5.1 atm; at
atmospheric
pressure, it passes directly between
the gaseous and solid phases
in a
process
called sublimation.
Synthetic
Strategies in Chemistry
14.3
7)
Water will absorb its own
volume of carbon dioxide, and
more than this
under
pressure.
About 1% of the dissolved
carbon dioxide turns into
carbonic acid. The
carbonic
acid in turn dissociates partly to
form bicarbonate and carbonate
ions.
Test
for Carbon dioxide
When
a lighted splint is inserted
into a test tube containing
carbon dioxide, the flame
is
immediately
extinguished, as carbon dioxide
does not support combustion.
(Certain fire
extinguishers
contain carbon dioxide to
extinguish the flame). To
further confirm that
the
gas
is carbon dioxide, the gas
may be bubbled into calcium
hydroxide solution.
The
calcium
hydroxide turns milky
because of the formation of
calcium carbonate.
Applications
1)
Liquid and solid carbon
dioxide are important
refrigerants, especially in the
food
industry,
where they are employed
during the transportation and
storage of ice
cream
and other frozen foods.
Solid carbon dioxide is
called "dry ice" and is
used
for
small shipments where
refrigeration equipment is not
practical.
2)
Carbon dioxide is used to
produce carbonated soft drinks and
soda water.
Traditionally,
the carbonation in beer and
sparkling wine come about
through
natural
fermentation, but some
manufacturers carbonate these
beverages
artificially.
3)
The leavening agents used in
baking produce carbon
dioxide to cause dough
to
rise.
Baker's yeast produces carbon dioxide by
fermentation within the
dough,
while
chemical leaveners such as
baking powder and baking
soda release carbon
dioxide
when heated or exposed to
acids.
4)
Carbon dioxide is often used
as an inexpensive, nonflammable
pressurized gas.
Life
jackets often contain canisters of
pressured carbon dioxide for
quick
inflation.
Steel capsules are also sold as
supplies of compressed gas
for air guns,
paintball
markers, for inflating
bicycle tires, and for
making seltzer. Rapid
vaporization
of liquid CO2 is
used for blasting in coal
mines.
5)
Carbon dioxide extinguishes
flames, and some fire
extinguishers, especially those
designed
for electrical fires;
contain liquid carbon
dioxide under pressure.
Carbon
dioxide
also finds use as an atmosphere for
welding, although in the
welding arc,
it
reacts to oxidize most
metals. Use in the automotive
industry is common
despite
14.4
Synthesis
of Chemicals from Carbon
dioxide
significant
evidence that welds made in
carbon dioxide are quite
delicate than
those
made in more inert atmospheres, and
that such weld joints
depreciate over
time
because of the formation of
carbonic acid. It is used as a
welding gas
primarily
because it is less expensive
than more inert gases
such as argon or
helium.
6)
Liquid carbon dioxide is a good
solvent for many organic
compounds and it has
begun
to attract attention in the
pharmaceutical and other chemical
processing
industries
as a less toxic alternative to
more traditional solvents
such as organic
chlorides.
It's used by some dry
cleaners for this
reason.
7)
Plants require carbon
dioxide to conduct photosynthesis, and
greenhouses may
enrich
their atmospheres with
additional CO2 to
boost plant growth. It has
been
proposed
that carbon dioxide from
power generation be bubbled
into ponds to
grow
algae that could then be
converted into biodiesel
fuel. High levels of
carbon
dioxide
in the atmosphere effectively exterminate
many pests. Greenhouses will
raise
the level of CO2 to
10,000 ppm (1%) for several
hours to eliminate
pests
such
as whitefly, spider mites, and
others.
8)
In medicine, up to 5% carbon dioxide is
added to pure oxygen for
stimulation of
breathing
after apnea and to stabilize
the O2/CO2 balance
in blood.
9)
A common type of industrial
gas laser, the carbon
dioxide laser, uses
carbon
dioxide
as a medium.
10)
Carbon dioxide is commonly
injected into or adjacent to
producing oil wells.
It
will
act as both a pressurizing agent and,
when dissolved into the
underground
crude
oil, will significantly reduce its
viscosity, enabling the oil
to flow more
rapidly
through the earth to the
removal well. In mature oil
fields, extensive pipe
networks
are used to carry the
carbon dioxide to the
injection points.
Carbon
dioxide - Dry Ice
1)
Dry ice
is a generalized trademark for
solid ("frozen") carbon
dioxide. Prest Air
Devices,
a company formed in Long
Island City, New York in
1923, coined the
term
in 1925.
Synthetic
Strategies in Chemistry
14.5
2)
Dry ice
at normal pressures does not
melt into liquid carbon
dioxide but rather
sublimates
directly into carbon dioxide
gas at -78.5 °C (-109.3 °F).
Hence it is
called
"dry ice" as opposed to
normal "wet" ice (frozen
water).
3)
Compressing
carbon dioxide gas to a
liquid form, removing the
heat produced by
the
compression, and then letting
the liquid carbon dioxide
expand quickly
produce
dry ice. This expansion
causes a drop in temperature so
that some of the
CO2 freezes
into "snow", which is then
compressed into pellets or
blocks.
Supercritical
Carbon dioxide
Carbon
dioxide also could be used
more widely as a solvent and
for example
super
critical CO2 (the
state existing at 31.0C and
72.8 atm). Now a days Carbon
dioxide
could
be used more widely as a
solvent and for example
supercritical carbon
dioxide
offers
advantages in terms of stereo
chemical control, product
purification synthesizing
fine
chemicals and pharmaceuticals. People
are extracting caffeine from
coffee by using
supercritical
carbon dioxide. More over
the advantage of using CO2 is
oil gas recovery
and
ponds of genetically modified algae
that can convert power plant
CO2 into biodiesel.
As
noted above, CO2 is
generally considered to be a green or environmentally
benign
solvent
and is naturally abundant. CO2 has
been suggested as a sustainable
replacement
for
organic solvents in a number of
chemical processes and is currently
used in the dry
cleaning,
and in parts degreasing. While CO2 is
certainly not a panacea,
there are a
number
of characteristics, which suggest
that CO2 could
provide environmental and
economic
benefits. The nontoxic
nature of CO2 has
a number of advantages. For
example,
in food and pharmaceutical applications,
usage of CO2 greatly
reduces future
liability
costs and can also facilitate regulatory
approval of certain processes. An
example
is
the conversion of pharmaceuticals
into nanometer-size particles
for injectable use.
Another
instance in which supercritical
carbon dioxide could be advantageous is
in
situations
involving contact between
hydrophilic and hydrophobic solvents. In
this case,
the
mutual solubility of the two
phases is designed to be small.
However, some cross-
contamination
is inevitable, typically leading to a
costly remediation. The use
of CO2 as
the
hydrophobic phase produces contamination
that is both benign and
readily reversible.
Examples
include liquid-liquid extraction
between organic and aqueous
phases as well as
emulsion
polymerization of water-soluble monomers.
In applications where emissions
are
14.6
Synthesis
of Chemicals from Carbon
dioxide
unavoidable,
CO2 is
relatively benign to the
environment. Examples range from
use of
CO2 in enhanced
oil recovery to use as a
foaming agent or as the solvent in
dry cleaning.
Using
supercritical CO2 as
a solvent also has advantages
that arise from chemical
and/or
physical
properties. In reactions involving
gaseous reactants in liquid phases,
the use of
supercritical
CO2 with its ability to
dissolve large amounts of
most gases could
allow
kinetic
control of reactions as opposed to
limiting of reaction rates by
the transport of the
gaseous
reactant across the
gas-liquid interface. In reactions
where CO2 is
a reagent, its
use
as a solvent would also favor
the reaction. Carbon dioxide
may also offer
advantages
in
reactions such as free-radical
polymerizations and oxidations where a
chemically inert
solvent
is required.
CHEMICALS
SYNTHESIS FROM CARBON
DIOXIDE
It
is well known that CO2 is
very stable gas and highly
unreactive but plants are
utilizing
it
for example in the
photosynthesis of carbohydrate from
CO2. Can we also
find the
ways
to make chemicals from CO2 artificially?
Surely it will be an
environmentally
benign
route, and this process will lead to
green chemistry. The main
process which use
CO2 are
1)
Synthesis of urea.
2)
Synthesis of salicylic acid
3)
Synthesis of cyclic carbonate and
polycarbonate
4)
Synthesis of methanol.
Synthesis
of urea by using CO2 currently
is a well-established process. The
capacity is
approximately
90 million metric tons per
annum as per 1997 statistics. Other
reactions
are
in pilot plant scale.
Besides to these reactions
there are many reactions,
which utilize
CO2. It will be a
great feed stock for making
commodity chemicals, fuels and
materials. It
already
plays a major role for a
variety of applications. But
there are few catches.
One is
that
CO2 is
very stable, which means it
takes extra effort to
achieve the molecules so
that
it
will react. Professor Christopher. M. Rayner of
the University of Leeds, in
England,
has
been working on CO2 conversion.
He published a review article
recently on the
potential
of CO2 in
synthetic organic chemistry.
Approximately 115 million
metric tons
of
CO2 is
used annually by the global
chemical industries but
really that does
not
compare
to the approximate 24 billion
metric tons. Bulk chemicals
already produced
Synthetic
Strategies in Chemistry
14.7
routinely
from CO2 include
urea to make nitrogen fertilizers,
salicylic acid as a
pharmaceutical
ingredient, and polycarbonate based and
plastics. The simplest
reactions
of
CO2 are those in which it is
simply inserted into an X-H
bond. Examples are
the
insertion
of CO2 into
organic amines to afford carbamic
acids, which may be
converted
into
organic carbamates. More
recent examples include the
insertion of CO2 in
P-N bonds
of
P (NR2)3 compounds
to form P (NR2)(OCONR2)
compounds
and the reaction of
2
ammonium
carbamates (derived from
CO2)
with alkyl halides in the
presence of crown
ethers
to form useful urethane
intermediates. This is an example of
using CO2 to
replace
phosgene,
a highly toxic intermediate in
chemical synthesis. Reactions
are known in
which
CO2 undergoes
insertion into Sn-C bonds of
allyl tin compounds to
form
carboxylated
allyl derivatives and which
are catalyzed by Pd complexes.
Another
interesting
reaction is the insertion of
CO2 into alkanes such as
methane to form acetic
acid.
The
activation of a C-H bond and
CO2 insertion are much
fascinating .The
thermodynamics
of this reaction are
marginal; however, adjusting
the reaction
conditions
and
coupling this reaction with
energetically favorable product
processes could
improve
conversion
efficiencies. Carbonates, (RO)2CO, can also be prepared by
inserting CO2
into
O-H
bonds followed by dehydration or by
oxidative carboxylation of olefins.
This
synthetic
approach has the possibility of
providing a new route to
compounds that have
very
large potential markets.
Related reactions in which
CO2 is
incorporated into
product
molecules
without reduction have been
used in the synthesis of
polymers.
The
groups of Inoue and Kuran
performed initial work in
this area. In recent years,
a
number
of new catalysts have been
developed for co polymerization of
CO2 and
oxiranes
to
form polycarbonate. These studies have
increased the productivity of
this reaction by
100
times and have also expanded the range of
applicable monomers
(oxiranes).
Polypyrones
are another potentially
interesting new class of
polymer. It has been
prepared
from diacetylenes and CO2 in
the presence of Ni catalysts; a
related reaction is
the
telomerization of butadiene and CO2 to produce lactones.
Urethanes have also
been
prepared
by the reactions of dicarbamate ions
formed by insertion of CO2 into diamines,
followed
by Pd-catalyzed coupling to
1,4-dichloro-2-butene. Reductive
carboxylations in
which
the CO2 unit is incorporated into
the product are also
known.
14.8
Synthesis
of Chemicals from Carbon
dioxide
In
the case of alkynes and
olefins, electrochemical reductive
carboxylations result in
effective
addition of the formic acid
C-H bond to C-C double or
triple bonds. For
example,
building on the earlier
stoichiometric results of Hoberg,
Dunach and co-workers
used
Ni bipyridine complexes and sacrificial
Mg anodes to reductively couple
acetylene
and
CO2 to
form propenic acid. Similarly,
Sylvestri reported that the
reductive coupling
of
CO2 with styrene is catalyzed
by benzonitrile. Bromoarenes can also be
reductively
carboxylated
to form the corresponding
carboxylic acid using Ni diphosphine
catalysts.
More
recently, the sequential
reductive coupling of two
molecules of CO2 to
butadiene to
form
3-hexen- 1,6-dioic acid has
been reported. This approach
provides a new route to
a
Nylon
precursor. Another important
monomer, ethylene, can be prepared
by
electrochemical
reduction of CO2 in
aqueous solutions with
current efficiencies as high
as
48%.
The production of this
monomer by this remarkable
12-electrons reduction offers
a
potential
route to polyethylene from
CO2.
The preceding results
clearly indicate that
it
may
be possible to produce a large variety of
polymers in the future using
materials
derived
from CO2.
Under
oxidative conditions, CO2 may
react with olefins to give
cyclic carbonates
that
find
wide industrial applications. In
these transformations heterogeneous
catalysts are
currently
more promising and viable
than homogeneous ones. Another
potentially useful
reaction
of CO2 is
the dehydrogenation of hydrocarbons.
Examples are the
dehydrogenation
of ethyl benzene and propane over metal
oxides to form styrene
and
propene,
respectively. In these reactions, no
part of the CO2 molecule
is incorporated into
the
organic product, rather the
oxygen of CO2 serves
to remove two H atoms of
the
hydrocarbon.
CO2 is
currently used as an additive in
the synthesis of methanol
from CO
and
H2, and it is
believed that reduced forms of
CO2 are kinetically
important
intermediates
in this process. Recently,
efficient heterogeneous catalysts have
been
developed
for CO2 hydrogenation
to methanol. However, the
thermodynamics for
methanol
production from H2 and
CO2 are not as favorable as
that for production
of
methanol
from H2 and
CO. For example, at 200 °C
the equilibrium yield of
methanol
from
CO2 is
slightly less than 40%
while the yield from CO is
greater than 80%. The
reduction
of CO2 can be rendered more favorable by
the use of hybrid catalysts
that
dehydrate
methanol to form Dimethyl
ether. Other copper-zinc
based catalysts have
also
Synthetic
Strategies in Chemistry
14.9
been
used for methanol synthesis.
Fisher and Bell et
al. studied
Cu/ZrO2/SiO2 catalysts
by
in-situ
infrared
spectroscopy and suggested some
mechanism for the route
to
methanol.
Ethanol has also been
produced by the hydrogenation of
CO2.
This fuel is
attractive
because it has a somewhat
higher energy density than
methanol and it is not as
toxic.
However, the selectivity for
ethanol production is comparatively
low (<40%).
The
hydrogenation of CO2 to
methane and higher hydrocarbons is also
known. For C2
and
higher hydrocarbons, hybrid
catalysts such as
Cu-ZnO-Cr2O3 and
H-Y zeolite are
generally
used. Noyori et
al.
have carried out pioneering
work on the catalytic
synthesis
of
formic acid derivatives by CO2 hydrogenation,
together with other substrates,
in
supercritical
CO2. In
part because of the high
solubility of H2
in CO2, an
economical
synthesis
of dimethylformamide is achieved.
Homogeneous
catalysts are also known to mediate
the rapid hydrogenation of
CO2 to
formate,
because this reaction is not
thermodynamically preferential, amines
and
supercritical
CO2 have been used to
drive this reaction. Under
the appropriate conditions,
very
high turnover numbers and
rates can be achieved. For
example, Leitner et
al.
examined
complexes of the general
type [R2P-(X)-PR2]Rh-(hfacac)
(X = bridging group;
hfacac)
1,3-bis-(trifluoromethyl)-acetonylacetonate). All the
compounds are active
catalysts
for formic acid production
from H2 and
CO2, but the most
effective has X=
(CH2)4 and
R= cyclohexyl showing good results at 25
°C and 40 atm of 1:1 H2:
CO2. The
selectivity
to formic acid is nearly
100%.
Possible
pathways for the opposing
interaction of Low-Valent Catalysts
with Protons
or
CO2 of
the CO2
reduction
product observed. If the reduced form of
the catalysts reacts
with
CO2 to
form a M-CO2
complex,
protonation yields a metallo
carboxylic acid;
further
reaction
can then produce CO by C-O
bond cleavage to form hydroxide or
water. Thus,
reaction
of a reduced form of the catalyst
with CO2,
as opposed to protons, leads to
CO
formation.
If the reduced form of the
catalyst reacts with protons
to form a hydride
complex,
subsequent reaction of the hydride
with CO2 leads to formate
production. An
interesting
example of such selectivity is
CO2 electrochemical reduction
catalyzed by
polymeric
films based on
[Ru(N-N)(CO)2]n
(N-N=poly
pyridine ligand) in aqueous
media.
Deronzier and Ziessel et
al. found
that bipyridyl ligands with
electron
withdrawing
groups in the 4,4 positions
gave catalysts which are
highly selective for
14.10
Synthesis
of Chemicals from Carbon
dioxide
formate
at pH >5 while those derived from
the unsubstantiated 2, 2 -bipyridine or
the 4,
4
-dimethyl analogue primarily
give CO at pH > 7.165 Formate was
thought to arise
from
an intermediate metal hydride, whereas CO
was thought to arise from a
metallo
carboxylicacid
generated by carbonation of an
intermediate anion followed
by
protonation.
It is unusual for homogeneous catalysts
to form reduction products
that
require
more than two
electrons.
However,
Tanaka and co-workers, reported
that the formation of
glycolate
(HOCH2COO-), glyoxylate
(OCHCOO-), formic acid, formaldehyde, and
methanol as
CO2 reduction products using
[Ru(tpy)(bpy)(CO)]2+
complexes as
electro catalysts (bpy
=
2,2
-bipyridine, and tpy =2,2 :6
,2
-terpyridine).Although
turnover numbers were
not
given for these highly
reduced species, their formation
raises the exciting
possibility
that
a single-site catalyst can result in
multi electron reductions of
CO2 and
even C-C
bond
formation.
Tanaka
and Gibson et
al.
recently succeeded in isolating
key Ru C1 compounds with
polypyridine
ligands that are models
for catalytic intermediates.
Gibson et
al.
also
isolated
ReC1 complexes with
polypyridine ligands. The
importance of photochemistry in
reactions
of some have the Re and Ru
complexes has been
demonstrated. The
formation
of
formaldehyde has also been
reported in electrochemical CO2 reduction using
transition
metal
terpyridine complexes polymerized on
glassy carbon electrodes. The
relatively
mild
conditions and low over
potentials required for some
of the homogeneous
catalysts
make
them attractive for future
studies; however, a number of
barriers must be
defeated
before
useful catalysts are
available for fuel
production.
Photochemical
reduction of CO2 is one of the significant
reactions many of the
reactions
described above rely on energy
input either in the form of
reactive bonds
(alkenes,
alkynes, etc.), hydrogen, or
electricity. Photochemical systems have
been
studied
in an effort to develop systems capable
of directly reducing CO2 to fuels or
chemicals
using solar energy.
Transition-metal complexes have
been used as both
catalysts
and solar energy converters, since
they can absorb a
significant portion of
the
solar
spectrum, have long-lived
excited states are able to
promote the activation of
small
molecules,
and are forceful. Carbon
dioxide utilization by artificial
photo conversion
presents
a challenging alternative to thermal
hydrogenation reactions, which
require H2.
Synthetic
Strategies in Chemistry
14.11
The
systems studied for photochemical
CO2 reduction studies can be divided
into several
groups:
Ru(bpy)32+ both as a photo sensitizer
and as a catalyst. Ru(bpy)32+ as
a photo
sensitizer
and another metal complex as a
catalyst. ReX (CO) 3(bpy) or a similar
complex
as
a photosensitizers. Ru(bpy)32+ and Ru(bpy)32+
type
complexes as photo sensitizers
in
microheterogeneous
systems. Metalloporphyrins also act as a
photosensitizers and
catalyst.
Photochemical CO2 reduction is normally
carried out less than
1.0 atm CO2
at
room
temperature. Therefore, the
concentration of dissolved CO2 in the solution is
low
(e.g.,
0.28 M in CH3-CN,
0.03Min water). These systems produce
formate and CO as
products.
In the most efficient systems,
the total quantum yield
for all reduced
products
reaches
40%. In some cases with Ru
or Os colloid, CH4 is produced with a low
quantum
yield.
Under photochemical conditions,
the turnover number and the
turnover frequency
are
dependent on irradiation wavelength,
light intensity, irradiation
time, and catalyst
concentration,
and they have not been
optimized in most of the
photochemical
experiments
described. Typical turnover frequencies
for CO or HCOO- are between 1 and
10h-1, and
turnover numbers are
generally 100 or less. The
abovementioned molecular
sensitizers
can be replaced with semiconductor electrodes or
particles to achieve
light
harvesting.
These systems may use enzymes or
catalysts to promote electron
transfer
from
the semiconductor-solution interface to
CO2 or reduce
CO2 directly. Typically
these
reductions
require a potential bias in
addition to solar energy
input to achieve CO2
reduction
and electrode corrosions a major
concern. This corrosion can
some times be
defeated
using
high
CO2
pressures.
Fascinating
examples
of
stoichiometric
photochemical
reactions of CO2 promoted by metal
complexes have also been
reported.
Thus,
Aresta et
al. found
that CO2 can be incorporated into
cyclopropane to afford
butyrolactone.
Kubiak et
al. demonstrated
the reduction of CO2 to the radical anion
and
subsequent
coupling to cyclohexene by the
use of a Ni complex.
Ranyar
et
al. have
worked on the catalytic
processes for reducing
CO2 to
formic acid.
It
has potential of power fuel
cell for electricity
generation and automobiles and as
a
precursor
for other fuels, synthetic
chemicals and fibers, including
polymers.
Conversion
of CO2 to CO will be more challenging
process because CO can be
used
in
a host of organic synthesis and one of
the important feed stock in
the chemical industry
for
making higher hydrocarbon
through Fisher-Tropsch
Synthesis.
14.12
Synthesis
of Chemicals from Carbon
dioxide
Nicolas
Eghbali and his group
working on conversion of CO2 and
olefins into cyclic
carbonates
in water. Geoffrey W.Coates et al.
have
developed the catalyst to
incorporate
CO2 into polymers by using
β-diminate zinc acetate and salen cobalt
carboxylate
complexes.
These catalysts promote alternating co
polymerization of various epoxides
with
CO2 to make
biodegradable aliphatic polycarbonate.
The polymers which
contain
30-50%
CO2 by
weight have gas barrier and
degradation properties that make
them
attractive
for feed packaging foam-casting to make
automotive parts and electronic
processing
applications. These kinds of polymers
also can be used to replace
propylene
oxide
segments in polyurethane foams,
which would help cut
costs. The foams are
used
for
insulation and seat cushions,
among other
applications
About
150 million tons of plastics
produced globally in a year, and
most of it is non-
bio
degradable and from energy
intensive processes that use
petroleum-based feedstocks.
Coastes
et
al. working
on limeonene oxide derived
from citrus fruit waste as
a
potential
epoxide monomer for
co-polymerization with CO2.other
research in progress
involves
developing a catalytic system
that can use untreated
CO2 directly from
industrial
waste
streams to make polymer.
Artificial
bio inspired systems is far
less complicated and therefore
easier to study
than
natural photosynthesis, in which
sun light water and CO2 are converted into
O2 and
carbohydrates.
By
using inorganic material, people
are utilizing the carbon
dioxide. The reaction
of
CaCO3 and
CO2 in water to form Ca
(HCO3)
is
responsible for the fixation of
large
2
quantities
of CO2 in the oceans. However, it
is kinetically slow. Similarly,
CO2 can also
be
fixed
by
naturally occurring
minerals.
Even
though
the
reactions
are
thermodynamically
favorable, they are slow and
would need to be enhanced
kinetically
before
they could contribute
significantly to adjusting the
carbon balance. In addition,
this
would generally require
mining and processing enormous amounts of
materials to
store
relatively little CO2.Currently, large
quantities of CaCO3
are
converted into CaO
and
CO2 (which is released into
the atmosphere) in cement manufacture. If
a natural ore
could
be substituted for CaO, a
significant release of CO2 into the atmosphere
could, in
principle,
be avoided.
Synthetic
Strategies in Chemistry
14.13
Fujita
has found success by using
rhenium tri carbonyl
complexes to mediate CO2
reduction.
The researchers homed in on one
set of catalysts bearing
bipyridine (bpy)
ligands
which includes (bpy)
Re(CO)3 and its di rhenium analog.
But the reaction
rates
for
co production are slow due to
the stability of CO2
People
are working on CO2 → CO but ultimate target
is CO2 to Methanol that
could
be
used as a fuel.
All
Possible Reactions
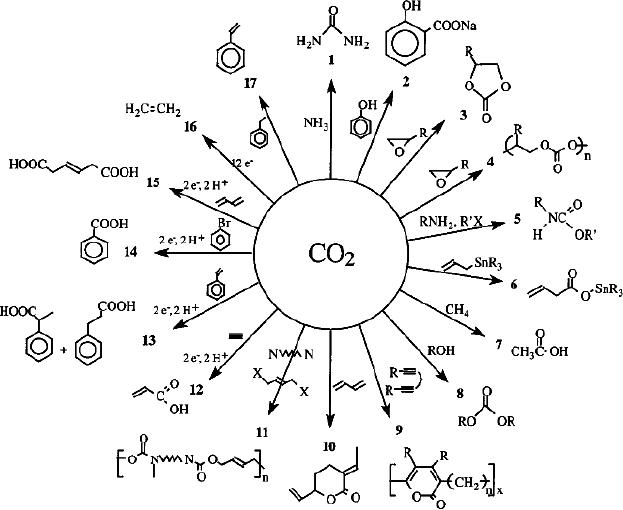
Fig.
14.1. Overall chemical
transformations
(reproduced
from Hironiri Arakawa, chemical
Reviews, 101 (2001)
976)
CONCLUSION
Synthesizing
chemicals from carbon
dioxide is one of the challenging
process as well as
eco-friendly
and environmentally benign route.
Using carbon dioxide as a
raw material
14.14
Synthesis
of Chemicals from Carbon
dioxide
never
going to reduce atmosphere CO2 level
or it will be very little the
effect on climate
change
but we can reduce the production of
CO2 and reduce
the usage of fossil fuel
and
shall
we make it?
REFERENCES
1.
J. M. Desimone, Science,
265 (1994)
359
2.
Hironiri Arakawa, chemical
Reviews,
101
(2001)
976
3.
Chemical & Engineering News,
April
30,
2007, 11
4.
Chemistry & Industry, July13, 2007,
13
5.
X. Xiaoding, J. A. Moulijin Energy and
Fuels, 10
(1996)
305
6.
Greenhouse
issues 1992
Feb
7.
M. A.Scibioh and B.Viswanathan
Proc.Indian
Natn Sci Acad.,
70 A (3) (2004) 407
Table of Contents:
- INTRODUCTION TO SYNTHETIC STRATEGIES IN CHEMISTRY:POROUS MATERIALS
- SYNTHETIC METHODS BASED ON ACTIVATING THE REACTANT:HALOGENATION OF BENZENE
- METHODS BASED ON ACTIVATING THE REACTING SUBSTANCE:Experimental method
- SYNTHESIS OF MATERIALS BASED ON SOLUBILITY PRINCIPLE
- SOL-GEL TECHNIQUES:DEFINITIONS, GENERAL MECHANISM, INORGANIC ROUTE
- TEMPLATE BASED SYNTHESISSynthesis, Mechanism and Pathway
- MICROEMULSION TECHNIQUES:Significance of Packing Parameter
- SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS
- NEWER SYNTHETIC STRATERGIES FOR NANOMATERIALS:Nanostructured Materials
- THE ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY:The Holy Bible
- ELECTROCHEMICAL SYNTHESIS:FEATURES OF ELECTROCHEMICAL SYNTHESIS
- NEWER REACTIONS AND PROCEDURES: CATALYTIC AND NONCATALYTIC
- SYNTHETIC STRATEGIES - FROM LABORATORY TO INDUSTRY
- SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice
- CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES
- SOME CONCEPTUAL DEVELOPMENTS IN SYNTHESIS IN CHEMISTRY
- COMPUTATIONAL BASICS UNDERLYING SYNTHETIC STRATEGIES